Purdue biologists clarify how a cellular ’spacecraft’ opens its airlock
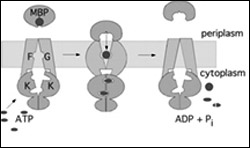
This graphic illustrates the process by which a membrane protein opens and closes, as envisioned by Jue Chen’s research team at Purdue University. ABC proteins, which are the inner portion of a membrane protein, function like tiny tweezers and are powered by ATP, a chemical that animal cells use for energy transport. When the tweezers squeeze shut, the outer section of the membrane protein opens to reveal a small cavity that can hold a nutrient or other substance the cell requires from the outside. Once the nutrient is there, the cell uses water to signal the "tweezers" to relax, closing the membrane protein gate and capturing the nutrient. Lastly, the membrane protein releases the nutrient into the cell’s interior. (Purdue graphic/Chen labs)
Scientists have a tough time visualizing the tiny hatchways that allow nutrients to pass into our cells, but a group of Purdue University biologists may have found the next best thing: a glimpse into the workings of the “motor” that opens and closes them.
A research team led by Jue Chen has clarified the connection between these minuscule gates – which are called membrane transport proteins – and the steps by which they use a cell’s energy to permit or deny materials entry into the interior of the cell from the outside world.
In what the team perceives to be a three-step process, cells feed chemical energy to a tiny machine called an ABC protein, which is the part of the membrane protein that connects it to the interior of the cell. These ABC proteins use the energy to bend the membrane protein into its open and closed positions, allowing the cell both to bring in nutrients and to flush out waste.
“We think we have a better handle on a process fundamental to life in creatures from bacteria to humans,” said Chen, who is an assistant professor of biology in Purdue’s College of Science. “This is the first time the entire cycle has been visualized, and this could enhance our understanding of how the process of metabolism unfolds.”
The team’s paper appears in this week’s issue of Proceedings of the National Academy of Sciences. Chen’s group also includes her Purdue colleagues Gang Lu and James M. Westbrooks, as well as Amy L. Davidson, who recently relocated to Purdue from the Baylor College of Medicine. The team used X-ray crystallography and other advanced imaging techniques to obtain a clear picture of the ABC protein, a method which has only had limited success in revealing secrets of the membrane proteins themselves.
Membrane proteins in cells have been likened to spacecraft airlocks, which ensure that only the astronauts gain entry and no air is lost. Where spacecraft have metal walls, cells have membranes that surround their inner protoplasm, and their airlock proteins are highly complex individual molecules that allow nutrients to enter cells and waste products to leave them.
Of the thousands of membrane proteins that exist, scientists only know the structure of a few dozen. They are of great interest to biologists because, as the regulators of intercellular commerce, they essentially permit metabolism – and, thus, life itself – to continue. However, while most proteins dissolve in water and can be easily crystallized and examined, membrane proteins dissolve only in fatty substances, making it difficult to isolate them for study.
“If we had a better understanding of this class of proteins, we might know more about how our bodies use and transfer energy,” Chen said. “It’s an unfortunate gap in our knowledge of how living things work. But in this study, we looked at a protein that is a bit of a hybrid: one part of it is fat-soluble, and the other is water-soluble.”
Because the entire membrane protein would not submit to crystallization, Chen’s team focused their efforts on the ATP-binding cassette proteins, or ABC proteins for short, that connect the membrane proteins with the cell’s interior. This portion of the protein is of the more study-friendly, water-soluble variety, and also plays a critical role in cellular commerce: It is the motor that drives a membrane protein’s motion.
“We isolated the ABC proteins from an E. coli bacterium, which is a very common research subject,” Chen said. “Different as these single-celled organisms are, their ABC proteins are structurally very similar to those in human cells, so studying them could help our knowledge of our own metabolism.”
ABC proteins function like tiny tweezers and are powered by ATP, a chemical that animal cells use for energy. When ATP causes the tweezers to squeeze shut, the membrane proteins open to reveal a small cavity that can hold a nutrient or other substance the cell requires from the outside. Once the nutrient is in place, the cell uses water to break down the ATP, signaling the “tweezers” to relax, closing the membrane protein gate and capturing the nutrient. Lastly, the membrane protein releases the nutrient into the cell’s interior.
“The ABC protein is like the inner door of the airlock; that’s what we were able to see in operation in this study,” Chen said. “If you opened both it and the membrane protein simultaneously, nothing would stop the interior of the cell from getting sucked out.”
Chen admits that the team is not yet certain that the description of the process is complete, though it does seem compelling based on what science already knows about the workings of membrane proteins.
“We need to look closer at our information and try to find out more,” Davidson said. “We will be applying several tests to our data in the near future to determine if our image of these proteins accurately describes their behavior.”
Chen said the work might have long-term payoffs in the fight against cancer, though it was too soon to make more than general statements as to how.
“Many cancer cells are resistant to anticancer drugs because the ABC proteins are overabundant and get too good at pumping the drugs out before they can work,” she said. “Future therapies might exploit what we are finding out about these proteins’ operation. It’s too soon to talk about specific therapies, but because there are so many kinds of cancer out there, every piece of knowledge helps.”
This research was sponsored in part by the National Institutes of Health and the Pew Charitable Trusts.
Members of Chen’s research group are associated with the Purdue Cancer Center. One of just seven National Cancer Institute-designated basic-research facilities in the United States, the center attempts to help cancer patients by identifying new molecular targets and designing future agents and drugs for effectively detecting and treating cancer. The Cancer Center is part of the Oncological Sciences Center in Purdue’s Discovery Park.
Writer: Chad Boutin, (765) 494-2081, cboutin@purdue.edu
Sources: Jue Chen, (765) 496-3113, chenjue@purdue.edu
Amy Davidson, (765) 494-5291, adavidso@purdue.edu
Purdue News Service: (765) 494-2096; purduenews@purdue.edu
Media Contact
More Information:
http://www.purdue.eduAll latest news from the category: Life Sciences and Chemistry
Articles and reports from the Life Sciences and chemistry area deal with applied and basic research into modern biology, chemistry and human medicine.
Valuable information can be found on a range of life sciences fields including bacteriology, biochemistry, bionics, bioinformatics, biophysics, biotechnology, genetics, geobotany, human biology, marine biology, microbiology, molecular biology, cellular biology, zoology, bioinorganic chemistry, microchemistry and environmental chemistry.
Newest articles

High-energy-density aqueous battery based on halogen multi-electron transfer
Traditional non-aqueous lithium-ion batteries have a high energy density, but their safety is compromised due to the flammable organic electrolytes they utilize. Aqueous batteries use water as the solvent for…

First-ever combined heart pump and pig kidney transplant
…gives new hope to patient with terminal illness. Surgeons at NYU Langone Health performed the first-ever combined mechanical heart pump and gene-edited pig kidney transplant surgery in a 54-year-old woman…

Biophysics: Testing how well biomarkers work
LMU researchers have developed a method to determine how reliably target proteins can be labeled using super-resolution fluorescence microscopy. Modern microscopy techniques make it possible to examine the inner workings…





















