Molecular motor Myosin VI moves ’hand over hand,’ researchers say
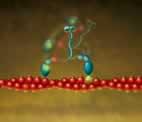
Myosin VI (blue) is a molecular motor that walks "backwards" on filaments of actin (red). By labeling a myosin VI on the head (green), or on the neck (red), and localizing the dye within a few nanometers, scientists determined that myosin walks "hand-over-hand," while causing a part of the protein to come undone. Graphic courtesy Paul Selvin
In the human body, hundreds of different types of biomolecular motors help carry out such essential tasks as muscle contraction, moving chromosomes during cell division, and reloading nerve cells so they can repeatedly fire.
How these little proteins perform their duties is becoming clearer to scientists using an extremely sensitive measurement technique. Myosin VI, they found, moves by the same “hand-over-hand” mechanism as two other molecular motors, myosin V and kinesin.
“Now that a third molecular motor has been found to move in the same hand-over-hand fashion, the argument for a rival ‘inchworm’ motion is getting pretty weak,” said Paul Selvin, a professor of physics at the University of Illinois at Urbana-Champaign and a co-author of a paper to appear in the Journal of Biological Chemistry.
Myosin VI is a reverse-direction molecular motor that moves materials to various locations within a living cell. Like the related protein myosin V, myosin VI has two “arms” connected to a “body.” The tiny molecule converts chemical energy into mechanical motion, and transports its load by “stepping” along polarized filaments of actin – but in the opposite direction from other myosin variants.
“Studies have suggested two main models for the stepping movement,” Selvin said. “One is the hand-over-hand model in which the two arms alternate in the lead. The other model is the inchworm model in which one arm always leads.”
To examine the myosin VI stepping mechanism, the researchers applied the same technique that was used to study both myosin V and kinesin. Called FIONA – Fluorescence Imaging with One Nanometer Accuracy – the measurement technique can track the position of a single molecule to within 1.5 nanometers. (One nanometer is a billionth of a meter, or about 10,000 times smaller than the width of a human hair).
“First, we attached a small fluorescent dye to one of the arms and took a picture with a digital camera attached to a microscope to find exactly where the dye was,” Selvin said. “Then we fed the myosin a little food called adenosine triphosphate, and it took a step. We took another picture, located the dye, and measured how far the dye moved.”
By examining the step size, the scientists could determine whether the protein used a hand-over-hand mechanism or an inchworm mechanism for movement. “The average step size for the myosin VI arm was approximately 60 nanometers, while the molecule’s center of mass moved only half that distance,” Selvin said. “This clearly indicated that a
hand-over-hand model was being employed.”
Surprisingly, myosin VI has a step size that is highly variable, but on average is nearly as large as that of myosin V, which has a lever arm that is three times longer. “For myosin VI to reach the same distance, the molecule must somehow come apart and then snap together again,” Selvin said. “To understand how it accomplishes this feat will require further study.”
The co-authors of the paper are Selvin, Hyokeun Park and Ahmet Yildiz at Illinois, and Li-Qiong Chen, Dan Safer, H. Lee Sweeney and Zhaohui Yang at the University of Pennsylvania. The National Institutes of Health funded the work.
Media Contact
More Information:
http://www.uiuc.eduAll latest news from the category: Life Sciences and Chemistry
Articles and reports from the Life Sciences and chemistry area deal with applied and basic research into modern biology, chemistry and human medicine.
Valuable information can be found on a range of life sciences fields including bacteriology, biochemistry, bionics, bioinformatics, biophysics, biotechnology, genetics, geobotany, human biology, marine biology, microbiology, molecular biology, cellular biology, zoology, bioinorganic chemistry, microchemistry and environmental chemistry.
Newest articles

High-energy-density aqueous battery based on halogen multi-electron transfer
Traditional non-aqueous lithium-ion batteries have a high energy density, but their safety is compromised due to the flammable organic electrolytes they utilize. Aqueous batteries use water as the solvent for…

First-ever combined heart pump and pig kidney transplant
…gives new hope to patient with terminal illness. Surgeons at NYU Langone Health performed the first-ever combined mechanical heart pump and gene-edited pig kidney transplant surgery in a 54-year-old woman…

Biophysics: Testing how well biomarkers work
LMU researchers have developed a method to determine how reliably target proteins can be labeled using super-resolution fluorescence microscopy. Modern microscopy techniques make it possible to examine the inner workings…





















