Prickly protein
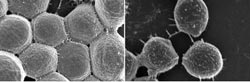
Images taken with a scanning electron microscope show wild-type bacteria (left) forming tight aggregates or clumps in the presence of blood proteins. In contrast, cells of the mutant strain (right) over produce a giant surface protein, have a spiky appearance, and do not clump tightly together. This clumping defect makes the mutant strain less deadly in an experimental model of the serious staph infection, endocarditis.<br><br>Credit: Alexander Horswill, University of Iowa<br>
A genetic mechanism that controls the production of a large spike-like protein on the surface of Staphylococcus aureus (staph) bacteria alters the ability of the bacteria to form clumps and to cause disease, according to a new University of Iowa study.
The new study is the first to link this genetic mechanism to the production of the giant surface protein and to clumping behavior in bacteria. It is also the first time that clumping behavior has been associated with endocarditis, a serious infection of heart valves that kills 20,000 Americans each year. The findings were published in the Dec. 2103 issue of the journal PLOS Pathogens.
Under normal conditions, staph bacteria interact with proteins in human blood to form aggregates, or clumps. This clumping behavior has been associated with pathogenesis — the ability of bacteria to cause disease. However, the mechanisms that control clumping are not well understood.
In the process of investigating how staph bacteria regulate cell-to-cell interactions, researchers at the UI Carver College of Medicine discovered a mutant strain of staph that does not clump at all in the presence of blood proteins.
Further investigation revealed that the clumping defect is due to disruption of a genetic signaling mechanism used by bacteria to sense and respond to their environment. The study shows that when the mechanism is disrupted, the giant surface protein is overproduced — giving the cells a spiny, or “porcupine-like” appearance — and the bacteria lose their ability to form clumps.
Importantly, the researchers led by Alexander Horswill, PhD, associate professor of microbiology, found that this clumping defect also makes the bacteria less dangerous in an experimental model of the serious staph infection, endocarditis.
Specifically, the team showed that wild type bacteria cause much larger vegetations (aggregates of bacteria) on the heart valves and are more deadly than the mutant bacteria, which are unable to form clumps. The experimental model of the disease was a good parallel to the team's test tube experiments.
“The mutant bacteria that don't clump in test tube experiments, don't form vegetations on the heart valves,” Horswill explains.
The team then created a version of the mutant bacteria that was also unable to make the giant surface protein. This strain regained the ability of form clumps and also partially regained its ability to cause disease, suggesting that the surface protein is at least partly responsible for both preventing clump formation and for reducing pathogenesis.
“Our study suggests that clumping could be a target for therapy,” says Horswill. “If we could find drugs that block clumping, I think they would be potentially really useful for blocking staph infections.”
Staph bacteria are the most significant cause of serious infectious disease in the United States, according to the Centers for Disease Control and Prevention (CDC). The bacteria are responsible for life-threatening conditions, including endocarditis, pneumonia, toxic shock, and sepsis. A better understanding of how staph bacteria causes disease may help improve treatment.
The team is now using screening methods to find small molecules that can block clumping. Such molecules will allow the researchers to investigate the clumping mechanism more thoroughly and may also point to therapies that might reduce the illness caused by staph infections.
The research was partially supported by grant funding from the National Institutes of Health (AI083211 and AI157153).
In addition to Horswill, the research team included Jeffrey Boyd, PhD, a former post doctoral researcher at the UI, whose early work initiated the study, and Patrick Schlievert, PhD, UI professor and chair of microbiology. UI scientists Jennifer Walker, Heidi Crosby, Adam Spaulding, Wilmara Salgado-Pabon, Cheryl Malone, and Carolyn Rosenthal were also part of the research team.
Media Contact
More Information:
http://www.uiowa.eduAll latest news from the category: Life Sciences and Chemistry
Articles and reports from the Life Sciences and chemistry area deal with applied and basic research into modern biology, chemistry and human medicine.
Valuable information can be found on a range of life sciences fields including bacteriology, biochemistry, bionics, bioinformatics, biophysics, biotechnology, genetics, geobotany, human biology, marine biology, microbiology, molecular biology, cellular biology, zoology, bioinorganic chemistry, microchemistry and environmental chemistry.
Newest articles

A universal framework for spatial biology
SpatialData is a freely accessible tool to unify and integrate data from different omics technologies accounting for spatial information, which can provide holistic insights into health and disease. Biological processes…

How complex biological processes arise
A $20 million grant from the U.S. National Science Foundation (NSF) will support the establishment and operation of the National Synthesis Center for Emergence in the Molecular and Cellular Sciences (NCEMS) at…

Airborne single-photon lidar system achieves high-resolution 3D imaging
Compact, low-power system opens doors for photon-efficient drone and satellite-based environmental monitoring and mapping. Researchers have developed a compact and lightweight single-photon airborne lidar system that can acquire high-resolution 3D…





















