New peptides to fight ovarian cancer drug resistance
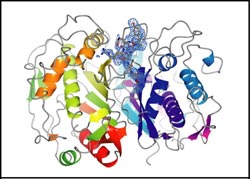
Structure of human thymidylate synthase with an inhibitory peptide bound at its dimer interface determined by x-ray crystallography. The protein is shown with a cartoon representation of its secondary structure colored according to sequence and the peptide is colored by atom-type with its electron density contoured in blue. Picture: Cardinale et al., PNAS (2011) 27 July 2011
The peptides act by a novel inhibitory mechanism and curb cancer cell growth in drug resistant ovarian cancer cells. The interdisciplinary research project was led by the University of Modena and Reggio Emilia (UNIMORE) and the Heidelberg Institute for Theoretical Studies (HITS).
Worldwide, over 200,000 women are diagnosed with ovarian cancer every year, with higher incidence in developed countries where it is the fifth leading cause of cancer-related deaths in women. Ovarian cancer has a high mortality rate due to frequent late diagnosis and the rapid development of drug resistance. Several clinically important anti-cancer drugs that are widely used in chemotherapy inhibit the enzyme, thymidylate synthase, which plays a key role in DNA synthesis. However, the use of these drugs is associated with drug resistance and new compounds with different inhibitory mechanisms are required to combat resistance.
Scientists from Italy and Germany have designed octapeptides that specifically target the protein-protein interface of thymidylate synthase. Thymidylate synthase is composed of two identical polypeptide chains, i.e. it is a homodimer. The peptides stabilize the inactive form of the enzyme, show a novel mechanism of inhibition for homodimeric enzymes, and inhibit cell growth in drug sensitive and resistant cancer cell lines.
The interdisciplinary collaboration between scientists in Italy and Germany, led by Maria Paola Costi and Glauco Ponterini at the University of Modena and Reggio Emilia, Stefano Mangani at the University of Siena (UNISI) and Rebecca Wade at Heidelberg Institute for Theoretical Studies (HITS), was part of the LIGHTS project (LIGands to interfere with human TS). The project was supported by the Sixth Framework Programme (FP6), an EU scheme to fund and promote European research and technological development.
The researchers have discovered several peptides that inhibit thymidylate synthase by modulating protein-protein interactions. Maria Paola Costi explains: “These peptides have sequences from the protein-protein interface of the enzyme and inhibit it by binding to a previously unknown allosteric binding site – that is, a site other than the protein's active site – at the protein-protein interface.” By a combination of experimental and computational approaches, it was shown that their inhibitory mechanism involving stabilization of an inactive form of the catalytic protein differs from those of protein-protein interface inhibitors reported to date.
Unlike the existing drugs targeting thymidylate synthase, these peptides inhibit intra-cellular thymidylate synthase and cell growth without leading to increased levels of thymidylate synthase protein when administered to ovarian cancer cells. “This observation indicates the potential value of these peptides in overcoming drug resistance problems, although the cellular effects remain to be fully explored,” says Rebecca Wade. Further steps will require optimization of the compounds discovered and detailed analysis of their cellular mechanism of action. The concepts revealed by this work can be expected to provide new avenues for the development of drugs for combating diseases such as ovarian cancer.
The original scientific article:
Cardinale et al., Protein-protein interface-binding peptides inhibit the cancer therapy target human thymidylate synthase. PNAS (2011) 27 July 2011 (published online before print).
doi: 10.1073/pnas.1104829108
http://www.pnas.org/cgi/doi/10.1073/pnas.1104829108
Press Contact
Dr. Peter Saueressig
Public Relations
Heidelberg Institute for Theoretical Studies (HITS)
Phone: +49-6221-533-245
Fax: +49-6221-533-198
peter.saueressig@h-its.org
www.h-its.org
Scientific Contacts
Prof. Maria Paola Costi
Dipartimento di Scienze Farmaceutiche,
Universita degli Studi di Modena e Reggio Emilia,
Via Campi 183,
41100 Modena,
Italy
Phone 0039-0592055125
Fax 0039-0592055131
mariapaola.costi@unimore.it
Prof. Stefano Mangani
Dipartimento di Chimica,
Università degli Studi di Siena,
Via Aldo Moro 2,
53100 Siena,
Italy
Phone 0039-0577-234255
Fax 0039-0577-243233
mangani@unisi.it
Dr. Rebecca Wade
Molecular and Cellular Modeling Group
Heidelberg Institute for Theoretical Studies (HITS)
Schloss-Wolfsbrunnenweg 35
69118 Heidelberg
Germany
Phone: +49 (0)6221 – 533 – 247
Fax: +49 (0)6221 – 533 – 298
rebecca.wade@h-its.org
University of Modena and Reggio Emilia (UNIMORE) is one of the oldest universities in Europe and currently has more than 20,000 students. Eight of the twelve faculties are located in Modena, among them the Biotechnology and Bioscience faculty with the Department of Pharmaceutical Sciences.
www.unimore.it
University of Siena (UNISI) is one of the oldest universities in Italy and currently has around 20,000 students. The university has nine schools, one of them being the School of Mathematical, Physical and Natural Sciences with the Department of Chemistry.
http://www.unisi.it
HITS (Heidelberg Institute for Theoretical Studies) is a private, non-profit research institute carrying out multidisciplinary research in the computational sciences. It was established in 2010 as a successor to the EML Research. HITS receives its base funding from the Klaus Tschira Foundation, which was established in 1995.
www.h-its.org
Media Contact
More Information:
http://www.h-its.orgAll latest news from the category: Life Sciences and Chemistry
Articles and reports from the Life Sciences and chemistry area deal with applied and basic research into modern biology, chemistry and human medicine.
Valuable information can be found on a range of life sciences fields including bacteriology, biochemistry, bionics, bioinformatics, biophysics, biotechnology, genetics, geobotany, human biology, marine biology, microbiology, molecular biology, cellular biology, zoology, bioinorganic chemistry, microchemistry and environmental chemistry.
Newest articles

High-energy-density aqueous battery based on halogen multi-electron transfer
Traditional non-aqueous lithium-ion batteries have a high energy density, but their safety is compromised due to the flammable organic electrolytes they utilize. Aqueous batteries use water as the solvent for…

First-ever combined heart pump and pig kidney transplant
…gives new hope to patient with terminal illness. Surgeons at NYU Langone Health performed the first-ever combined mechanical heart pump and gene-edited pig kidney transplant surgery in a 54-year-old woman…

Biophysics: Testing how well biomarkers work
LMU researchers have developed a method to determine how reliably target proteins can be labeled using super-resolution fluorescence microscopy. Modern microscopy techniques make it possible to examine the inner workings…





















