New class of DNA repair enzyme discovered
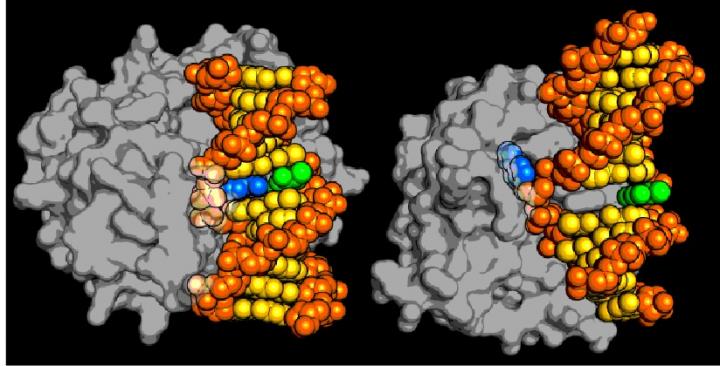
The new type of DNA repair enzyme, AlkD on the left, can identify and remove a damaged DNA base without forcing it to physically "flip" to the outside of the DNA backbone, which is how all the other DNA repair enzymes in its family work, as illustrated by the human AAG enzyme on the right. The enzymes are shown in grey, the DNA backbone is orange, normal DNA base pairs are yellow, the damaged base is blue and its pair base is green. Credit: Brandt Eichman, Vanderbilt University
When the structure of DNA was first discovered, scientists imagined it to be extremely chemically stable, which allowed it to act as a blueprint for passing the basic traits of parents along to their offspring.
Although this view has remained prevalent among the public, biologists have since learned that the double helix is in fact a highly reactive molecule that is constantly being damaged and that cells must make unceasing repair efforts to protect the genetic information that it contains.
“It's a double-edged sword,” said Brandt Eichman, associate professor of biological sciences and biochemistry at Vanderbilt University, who headed the research team that made the new discovery. “If DNA were too reactive then it wouldn't be capable of storing genetic information. But, if it were too stable, then it wouldn't allow organisms to evolve.”
The DNA double-helix has a spiral staircase structure with the outer edges made from sugar and phosphate molecules joined by stair steps composed of pairs of four nucleotide bases (adenine, cytosine, guanine and thymine) that serve as the basic letters in the genetic code.
There are two basic sources of DNA damage or lesions: environmental sources including ultraviolet light, toxic chemicals and ionizing radiation and internal sources, including a number of the cell's own metabolites (the chemicals it produces during normal metabolism), reactive oxygen species and even water.
“More than 10,000 DNA damage events occur each day in every cell in the human body that must be repaired for DNA to function properly,” said first author Elwood Mullins, a postdoctoral research associate in the Eichman lab.
The newly discovered DNA repair enzyme is a DNA glycosylase, a family of enzymes discovered by Tomas Lindahl, who received this year's Nobel prize for recognizing that these enzymes removed damaged DNA bases through a process called base-excision repair. It was the first of about 10 different DNA repair pathways that biologists have identified to date.
In base-excision repair, a specific glycosylase molecule binds to DNA at the location of a lesion and bends the double-helix in a way that causes the damaged base to flip from the inside of the helix to the outside. The enzyme fits around the flipped out base and holds it in a position that exposes its link to the DNA's sugar backbone, allowing the enzyme to detach it. After the damaged base has been removed, additional DNA-repair proteins move in to replace it with a pristine base.
Eichman and his collaborators discovered that a glycosylase called AlkD found in Bacillus cereus – a soil-dwelling bacterium responsible for a type of food poisoning called the “fried rice syndrome” – works in a totally different fashion. It does not require base flipping to recognize damaged DNA or repair it.
Seven years ago, Eichman's group discovered that AlkD had a structure unlike any of the other glycosylases. The researchers determined that the enzyme was able to locate damaged DNA that has a positive electrical charge. This is the signature of alkylation, attaching chains of carbon and hydrogen atoms of varying lengths (methyl, ethyl etc.), to specific positions on the damaged base. Positively charged alkylated bases are among the most abundant and detrimental forms of DNA damage. However, they are highly unstable, which has made them very difficult to study.
Now the researchers have captured crystallographic snapshots of AlkD in the act of excising alkylation damage and have shown that the enzyme doesn't use base flipping. Instead, they have determined that AlkD forms a series of interactions with the DNA backbone at and around the lesion while the lesion is still stacked in the double helix. Several of these interactions are contributed by three amino acids in the enzyme that catalyze excision of the damaged base.
According to the researchers, the AlkD mechanism has some remarkable properties:
- It can recognize damaged bases indirectly. AlkD identifies lesions by interacting with the DNA backbone without contacting the damaged base itself.
- It can repair many different types of lesions as long as they are positively charged. By contrast, the base-flipping mechanism used by other glycosylases relies on a relatively tight binding pocket in the enzyme, so each glycosylase is designed to work with a limited number of lesions. AlkD doesn't have the same type of pocket so it isn't restricted in the same way. Instead, the catalytic mechanism that AlkD uses is limited to removing positively charged lesions.
- It can excise much bulkier lesions than other glycosylases. Base excision repair is generally limited to relatively small lesions. A different pathway, called nucleotide excision repair, handles larger lesions like those caused by UV radiation damage. However, Eichman's team discovered that AlkD could excise extremely bulky lesions, such as the one caused by the antibiotic yatakemycin, which is beyond the capability of other glycosylases.
“Our discovery shows that we still have a lot to learn about DNA repair, and that there may be alternative repair pathways yet to be discovered. It certainly shows us that a much broader range of DNA damage can be removed in ways that we didn't think were possible,” said Eichman. “Bacteria are using this to their advantage to protect themselves against the antibacterial agents they produce. Humans may even have DNA-repair enzymes that operate in a similar fashion to remove complex types of DNA damage. This could have clinical relevance because these enzymes, if they exist, could be reducing the effectiveness of drugs designed to kill cancer cells by shutting down their ability to replicate.”
###
Co-authors of the paper are graduate student Rongxin Shi and Postdoctoral Research Associate Zack Parsons from the Eichman Lab, Professor Sheila David and graduate student Philip Yuen from University of California, Davis and Professor Yasuhiro Igarashi from Toyama Prefectural University.
The research was supported by National Science Foundation grants MCB-1122098 and MCB-1517695 and National Institutes of Health grants R01ES01625 and R01CA067985.
Media Contact
All latest news from the category: Life Sciences and Chemistry
Articles and reports from the Life Sciences and chemistry area deal with applied and basic research into modern biology, chemistry and human medicine.
Valuable information can be found on a range of life sciences fields including bacteriology, biochemistry, bionics, bioinformatics, biophysics, biotechnology, genetics, geobotany, human biology, marine biology, microbiology, molecular biology, cellular biology, zoology, bioinorganic chemistry, microchemistry and environmental chemistry.
Newest articles

High-energy-density aqueous battery based on halogen multi-electron transfer
Traditional non-aqueous lithium-ion batteries have a high energy density, but their safety is compromised due to the flammable organic electrolytes they utilize. Aqueous batteries use water as the solvent for…

First-ever combined heart pump and pig kidney transplant
…gives new hope to patient with terminal illness. Surgeons at NYU Langone Health performed the first-ever combined mechanical heart pump and gene-edited pig kidney transplant surgery in a 54-year-old woman…

Biophysics: Testing how well biomarkers work
LMU researchers have developed a method to determine how reliably target proteins can be labeled using super-resolution fluorescence microscopy. Modern microscopy techniques make it possible to examine the inner workings…





















