Listen! How nerve cells flexibly adapt to acoustic signals
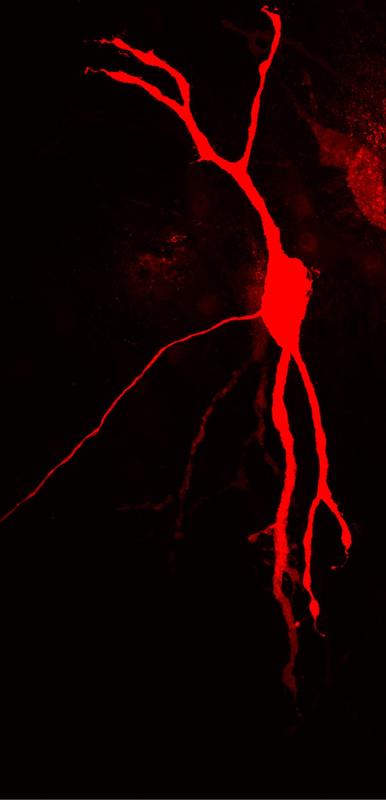
A neuron in the brain stem, that processes acoustic information. Depending on the situation, the cell generates action potentials in the axon (thin process) either close to or far from the body. Felix Felmy, 2014
In order to process acoustic information with high temporal fidelity, nerve cells may flexibly adapt their mode of operation according to the situation. At low input frequencies, they generate most outgoing action potentials close to the cell body.
Following inhibitory or high frequency excitatory signals, the cells produce many action potentials more distantly. This way, they are highly sensitive to the different types of input signals.
These findings have been obtained by a research team headed by Professor Christian Leibold, Professor Benedikt Grothe, and Dr. Felix Felmy from the Bernstein Center and the Bernstein Focus Neurotechnology in Munich and the LMU Munich, who used computer models in their study. The researchers report their results in the latest issue of The Journal of Neuroscience.
Did the bang come from ahead or from the right? In order to localize sound sources, nerve cells in the brain stem evaluate the different arrival times of acoustic signals at the two ears. Being able to detect temporal discrepancies of up to 10 millionths of a second, the neurons have to become excited very quickly. In this process, they change the electrical voltage that prevails on their cell membrane.
If a certain threshold is exceeded, the neurons generate a strong electrical signal—a so-called action potential—which can be transmitted efficiently over long axon distances without weakening. In order to reach the threshold, the input signals are summed up. This is achieved easier, the slower the nerve cells alter their electrical membrane potential.
These requirements—rapid voltage changes for a high temporal resolution of the input signals, and slow voltage changes for an optimal signal integration that is necessary for the generation of an action potential—represent a paradoxical challenge for the nerve cell. “This problem is solved by nature by spatially separating the two processes. While input signals are processed in the cell body and the dendrites, action potentials are generated in the axon, a cell process,” says Leibold, leader of the study. But how sustainable is the spatial separation?
In their study, the researchers measured the axons’ geometry and the threshold of the corresponding cells and then constructed a computer model that allowed them to investigate the effectiveness of this spatial separation. The researchers’ model predicts that depending on the situation, neurons produce action potentials with more or less proximity to the cell body.
For high frequency or inhibitory input signals, the cells will shift the location from the axon’s starting point to more distant regions. In this way, the nerve cells ensure that the various kinds of input signals are optimally processed—and thus allow us to perceive both small and large acoustic arrival time differences well, and thereby localize sounds in space.
The Bernstein Center Munich is part of the National Bernstein Network Computational Neuroscience in Germany. With this funding initiative, the German Federal Ministry of Education and Research (BMBF) has supported the new discipline of Computational Neuroscience since 2004 with over 170 million Euros. The network is named after the German physiologist Julius Bernstein (1835-1917).
Contact:
Prof. Dr. Christian Leibold
Computational Neuroscience
Department Biology II
LMU Munich
Großhaderner Straße 2
82152 Planegg-Martinsried (Germany)
Tel: +49 (0)89 2180-74802
Email: leibold@bio.lmu.de
Original publication:
S. Lehnert, M. C. Ford, O. Alexandrova, F. Hellmundth, F. Felmy, B. Grothe & C. Leibold (2014): Action potential generation in an anatomically constrained model of medial superior olive axons. Journal of Neuroscience, 34(15): 5370—5384.
http://neuro.bio.lmu.de/research_groups/res-leibold_ch personal website Christian Leibold
http://www.bccn-munich.de Bernstein Center München
http://www.uni-muenchen.de LMU Munich
http://www.nncn.de National Bernstein Network Computational Neuroscience
Media Contact
All latest news from the category: Life Sciences and Chemistry
Articles and reports from the Life Sciences and chemistry area deal with applied and basic research into modern biology, chemistry and human medicine.
Valuable information can be found on a range of life sciences fields including bacteriology, biochemistry, bionics, bioinformatics, biophysics, biotechnology, genetics, geobotany, human biology, marine biology, microbiology, molecular biology, cellular biology, zoology, bioinorganic chemistry, microchemistry and environmental chemistry.
Newest articles

High-energy-density aqueous battery based on halogen multi-electron transfer
Traditional non-aqueous lithium-ion batteries have a high energy density, but their safety is compromised due to the flammable organic electrolytes they utilize. Aqueous batteries use water as the solvent for…

First-ever combined heart pump and pig kidney transplant
…gives new hope to patient with terminal illness. Surgeons at NYU Langone Health performed the first-ever combined mechanical heart pump and gene-edited pig kidney transplant surgery in a 54-year-old woman…

Biophysics: Testing how well biomarkers work
LMU researchers have developed a method to determine how reliably target proteins can be labeled using super-resolution fluorescence microscopy. Modern microscopy techniques make it possible to examine the inner workings…





















