Injectable sponge delivers drugs, cells, and structure
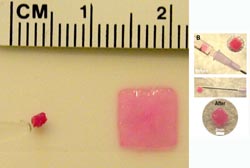
Left: A fully collapsed square-shaped cryogel rapidly regains its original memorized shape, size, and volume upon hydration. Right: Photos show the placement of a cryogel inside a 1-mL syringe, and the recovery of a square gel after injection through a normal 16-gauge needle. (Images courtesy of Sidi Bencherif.)<br>
Bioengineers at Harvard have developed a gel-based sponge that can be molded to any shape, loaded with drugs or stem cells, compressed to a fraction of its size, and delivered via injection. Once inside the body, it pops back to its original shape and gradually releases its cargo, before safely degrading.
The biocompatible technology, revealed this week in the Proceedings of the National Academy of Sciences, amounts to a prefabricated healing kit for a range of minimally invasive therapeutic applications, including regenerative medicine.
“What we’ve created is a three-dimensional structure that you could use to influence the cells in the tissue surrounding it and perhaps promote tissue formation,” explains principal investigator David J. Mooney, Robert P. Pinkas Family Professor of Bioengineering at the Harvard School of Engineering and Applied Sciences (SEAS) and a Core Faculty Member at the Wyss Institute for Biologically Inspired Engineering at Harvard.
“The simplest application is when you want bulking,” Mooney explains. “If you want to introduce some material into the body to replace tissue that’s been lost or that is deficient, this would be ideal. In other situations, you could use it to transplant stem cells if you’re trying to promote tissue regeneration, or you might want to transplant immune cells, if you’re looking at immunotherapy.”
Consisting primarily of alginate, a seaweed-based jelly, the injectable sponge contains networks of large pores, which allow liquids and large molecules to easily flow through it. Mooney and his research team demonstrated that live cells can be attached to the walls of this network and delivered intact along with the sponge, through a small-bore needle. Mooney’s team also demonstrated that the sponge can hold large and small proteins and drugs within the alginate jelly itself, which are gradually released as the biocompatible matrix starts to break down inside the body.
Normally, a scaffold like this would have to be implanted surgically. Gels can also be injected, but until now those gels would not have carried any inherent structure; they would simply flow to fill whatever space was available.
“Our scaffolds can be designed in any size and shape, and injected in situ as a safe, preformed, fully characterized, sterile, and controlled delivery device for cells and drugs,” says lead author Sidi Bencherif, a postdoctoral research associate in Mooney’s lab at SEAS and at the Wyss Institute.
Bencherif and his colleagues pushed pink squares, hearts, and stars through a syringe to demonstrate the versatility and robustness of their gel (see video).
The spongelike gel is formed through a freezing process called cryogelation. As the water in the alginate solution starts to freeze, pure ice crystals form, which makes the surrounding gel more concentrated as it sets. Later on, the ice crystals melt, leaving behind a network of pores. By carefully calibrating this mixture and the timing of the freezing process, Mooney, Bencherif, and their colleagues found that they could produce a gel that is extremely strong and compressible, unlike most alginate gels, which are brittle.
The resulting “cryogel” fills a gap that has previously been unmet in biomedical engineering.
“These injectable cryogels will be especially useful for a number of clinical applications including cell therapy, tissue engineering, dermal filler in cosmetics, drug delivery, and scaffold-based immunotherapy,” says Bencherif. “Furthermore, the ability of these materials to reassume specific, pre-defined shapes after injection is likely to be useful in applications such as tissue patches where one desires a patch of a specific size and shape, and when one desires to fill a large defect site with multiple smaller objects. These could pack in such a manner to leave voids that enhance diffusional transport to and from the objects and the host, and promote vascularization around each object.”
The next step in the team’s research is to perfect the degradation rate of the scaffold so that it breaks down at the same rate at which newly grown tissue replaces it. Harvard’s Office of Technology Development has filed patent applications on the invention and is actively pursuing licensing and commercialization opportunities.
Coauthors included R. Warren Sands, Deen Bhatta, and Catia S. Verbeke at SEAS; Praveen Arany at SEAS and the Wyss Institute; and David Edwards, who is Gordon McKay Professor of the Practice of Bioengineering at SEAS and a Core Faculty Member at the Wyss Institute.
The research was supported by the Wyss Institute for Biologically Inspired Engineering at Harvard, the National Institutes of Health, and the Juvenile Diabetes Research Foundation.
Supplemental videos are available, via PNAS, here:
http://www.pnas.org/content/suppl/2012/11/08/1211516109.DCSupplemental
PRESS CONTACTS:
Harvard School of Engineering and Applied Sciences
Caroline Perry, (617) 496-1351
Wyss Institute for Biologically Inspired Engineering at Harvard
Kristen Kusek, (617) 432-8266
Media Contact
More Information:
http://www.seas.harvard.eduAll latest news from the category: Life Sciences and Chemistry
Articles and reports from the Life Sciences and chemistry area deal with applied and basic research into modern biology, chemistry and human medicine.
Valuable information can be found on a range of life sciences fields including bacteriology, biochemistry, bionics, bioinformatics, biophysics, biotechnology, genetics, geobotany, human biology, marine biology, microbiology, molecular biology, cellular biology, zoology, bioinorganic chemistry, microchemistry and environmental chemistry.
Newest articles

A universal framework for spatial biology
SpatialData is a freely accessible tool to unify and integrate data from different omics technologies accounting for spatial information, which can provide holistic insights into health and disease. Biological processes…

How complex biological processes arise
A $20 million grant from the U.S. National Science Foundation (NSF) will support the establishment and operation of the National Synthesis Center for Emergence in the Molecular and Cellular Sciences (NCEMS) at…

Airborne single-photon lidar system achieves high-resolution 3D imaging
Compact, low-power system opens doors for photon-efficient drone and satellite-based environmental monitoring and mapping. Researchers have developed a compact and lightweight single-photon airborne lidar system that can acquire high-resolution 3D…





















