Artificial leaf jumps developmental hurdle
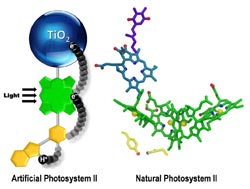
An artificial photosynthetic reaction center containing a bioinspired electron relay (yellow) mimics some aspects of photosynthesis. <br>
Designing an artificial leaf that uses solar energy to convert water cheaply and efficiently into hydrogen and oxygen is one of the goals of BISfuel – the Energy Frontier Research Center, funded by the Department of Energy, in the Department of Chemistry and Biochemistry at Arizona State University.
Hydrogen is an important fuel in itself and serves as an indispensible reagent for the production of light hydrocarbon fuels from heavy petroleum feed stocks. Society requires a renewable source of fuel that is widely distributed, abundant, inexpensive and environmentally clean.
Society needs cheap hydrogen.
“Initially, our artificial leaf did not work very well, and our diagnostic studies on why indicated that a step where a fast chemical reaction had to interact with a slow chemical reaction was not efficient,” said ASU chemistry professor Thomas Moore. “The fast one is the step where light energy is converted to chemical energy, and the slow one is the step where the chemical energy is used to convert water into its elements viz. hydrogen and oxygen.”
The researchers took a closer look at how nature had overcome a related problem in the part of the photosynthetic process where water is oxidized to yield oxygen.
“We looked in detail and found that nature had used an intermediate step,” said Moore. “This intermediate step involved a relay for electrons in which one half of the relay interacted with the fast step in an optimal way to satisfy it, and the other half of the relay then had time to do the slow step of water oxidation in an efficient way.”
They then designed an artificial relay based on the natural one and were rewarded with a major improvement.
Seeking to understand what they had achieved, the team then looked in detail at the atomic level to figure out how this might work. They used X-ray crystallography and optical and magnetic resonance spectroscopy techniques to determine the local electromagnetic environment of the electrons and protons participating in the relay, and with the help of theory (proton coupled electron transfer mechanism), identified a unique structural feature of the relay. This was an unusually short bond between a hydrogen atom and a nitrogen atom that facilitates the correct working of the relay.
They also found subtle magnetic features of the electronic structure of the artificial relay that mirrored those found in the natural system.
Not only has the artificial system been improved, but the team understands better how the natural system works. This will be important as scientists develop the artificial leaf approach to sustainably harnessing the solar energy needed to provide the food, fuel and fiber that human needs are increasingly demanding.
ASU chemistry professors involved in this specific project include Thomas Moore, Devens Gust, Ana Moore and Vladimiro Mujica. The department is a unit of the College of Liberal Arts and Sciences. Key collaborators in this work are Oleg Poluektov and Tijana Rajh from Argonne National Laboratory.
This work would not have been possible without the participation of many scientists driven by a common goal and coordinated by a program such as the Energy Frontier Research Center to bring the right combination of high-level skills to the research table.
The Department of Chemisry and Biocehmistry is an academic unit in ASU's College of Liberal Arts and Sciences.
Jenny Green, jenny.green@asu.edu
480-965-1430
Department of Chemistry and Biochemistry
Media Contact
More Information:
http://www.asu.eduAll latest news from the category: Life Sciences and Chemistry
Articles and reports from the Life Sciences and chemistry area deal with applied and basic research into modern biology, chemistry and human medicine.
Valuable information can be found on a range of life sciences fields including bacteriology, biochemistry, bionics, bioinformatics, biophysics, biotechnology, genetics, geobotany, human biology, marine biology, microbiology, molecular biology, cellular biology, zoology, bioinorganic chemistry, microchemistry and environmental chemistry.
Newest articles

A universal framework for spatial biology
SpatialData is a freely accessible tool to unify and integrate data from different omics technologies accounting for spatial information, which can provide holistic insights into health and disease. Biological processes…

How complex biological processes arise
A $20 million grant from the U.S. National Science Foundation (NSF) will support the establishment and operation of the National Synthesis Center for Emergence in the Molecular and Cellular Sciences (NCEMS) at…

Airborne single-photon lidar system achieves high-resolution 3D imaging
Compact, low-power system opens doors for photon-efficient drone and satellite-based environmental monitoring and mapping. Researchers have developed a compact and lightweight single-photon airborne lidar system that can acquire high-resolution 3D…





















