A view into the human body: the formation of cell organelles
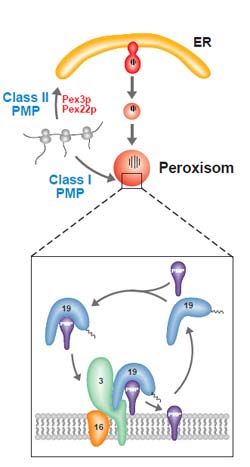
Pathways of peroxisomal membrane proteins: Type 1 peroxisomal membrane proteins (PMPs) are synthesized on free ribosomes within the cytosol and bound to the peroxisomal membrane immediately after formation. By contrast, the Type 2 PMPs, which, according to the data gained in this study, include Pex3p and Pex22p, are initially transported to the endoplasmic reticulum and only subsequently to the peroxisomes.<br>
Scientists at the RUB publish the results of their study in the Journal of Biological Chemistry
Peroxisomes, multifaceted functional units within cells, fulfil the most diverse of tasks. In human beings, failure of these organelles is fatal. Their biogenesis does not adhere to the standard rules and still remains to be fully clarified. Scientists from the research team under the auspices of Prof. Ralf Erdmann and Dr. Hanspeter Rottensteiner (Department of Systemic Biochemistry, Medical Faculty at the Ruhr University) have identified a new pathway for components of the peroxisomal membrane proteins.
The data gained is a significant contribution towards the comprehension of the formation and origin of peroxisomes. The results have been published in the renowned “Journal of Biological Chemistry.”
Peroxisomes: Organelles with multifaceted functions
Peroxisomes are organelles responsible for a multitude of metabolic functions within cells. They contain over 50 different functional enzymes capable of forming highly variable structures adapted to the specific needs of the organism. One of the most important features of peroxisomes is the spatial isolation (compartmentalization) of metabolic pathways in which poisonous hydrogen peroxide is formed. Its destruction thereof is one of the most important functions of the peroxisomes.
Illnesses due to peroxisomal defects are usually fatal
Prof. Erdmann explained that the significance of these organelles is illustrated by the illnesses of patients suffering from defects in individual enzymes or a disorder in the biogenesis of the peroxisomes. Illnesses resulting from a biogenetic disorder are subsumed as the Zellweger spectrum and are usually so severe that the patients die during infancy. Clarification of the biogenesis of these organelles is essential for the development of approaches for diagnosis and treatment of these diseases.
Indirect formation of membranes
The research team at the RUB investigated – amongst other things – the origin and biogenesis of the peroxisomal membranes. Prof. Erdmann explained that the peroxisomal protein import machinery has to be assembled before the peroxisome can import the numerous enzymes required. The Pex3p protein is a decisive factor for importing membrane proteins, functioning as docking site on the membrane. These docking sites enable targeted insertion of newly formed proteins. To date, it has still not been fully clarified how Pex3p per se is integrated into the membrane. It could, however, be shown that newly formed Pex3p is initially incorporated in the cellular fluid of the endoplasmic reticulum (ER, another cell organelle). The subsequent mode of transport of Pex3p to the peroxisomes is still unknown. Prof. Erdmann stated that there is a significant difference between this pathway and that of other peroxisomal membrane proteins. The latter are incorporated directly into existent peroxisomes and require Pex3p for this procedure.
Assumed exclusive pathway transpired to be common
To date it has been assumed that Pex3p is the only peroxisomal protein that reaches the peroxisomes via the endoplasmic reticulum. The scientists at the RUB have now been able to prove that this is not the case. They compared the import pathways of Pex3p with those of Pex22p, another peroxisomal membrane protein, and were able to demonstrate that in each case a small fraction of these proteins already suffices to transport a fluorescent reporter protein to the peroxisomes. The segments of the protein that target a specific site are termed signal sequences. The similarity between Pex3p and Pex22p signal sequences induced the scientists to exchange the two parts using molecular biological techniques and to subsequently investigate the functionality of the two thus altered proteins. Their analyses disclosed that the signal sequences of the two proteins are interchangeable without affecting their peroxisomal targeting. Further investigations showed that both proteins use the same pathway. This implies that this pathway is not exclusively used by Pex3p (as assumed to date) but rather constitutes a newly identified general pathway for peroxisomal membrane proteins. Moreover, this study showed that the Pex3p-signal sequence is responsible for targeting, but not – in contrast to the generally accepted opinion – for the specific function of Pex3p in the biogenesis of the peroxisomes.
Title
André Halbach, Robert Rucktäschel, Hanspeter Rottensteiner, Ralf Erdmann: The N-domain of Pex22p Can Functionally Replace the Pex3p N-domain in Targeting and Peroxisome Formation. In: The Journal of Biological Chemistry, Vol. 284, Issue 6, 3906-3916, FEBRUARY 6, 2009
Further Information
Prof. Dr. Ralf Erdmann, Department of Systemic Biochemistry at the Ruhr-University Bochum, 44780 Bochum, Tel. +49(0)234/32-24943, E-Mail: Ralf.Erdmann@rub.de
Editorial Staff: Meike Drießen
Media Contact
More Information:
http://www.ruhr-uni-bochum.de/All latest news from the category: Life Sciences and Chemistry
Articles and reports from the Life Sciences and chemistry area deal with applied and basic research into modern biology, chemistry and human medicine.
Valuable information can be found on a range of life sciences fields including bacteriology, biochemistry, bionics, bioinformatics, biophysics, biotechnology, genetics, geobotany, human biology, marine biology, microbiology, molecular biology, cellular biology, zoology, bioinorganic chemistry, microchemistry and environmental chemistry.
Newest articles

High-energy-density aqueous battery based on halogen multi-electron transfer
Traditional non-aqueous lithium-ion batteries have a high energy density, but their safety is compromised due to the flammable organic electrolytes they utilize. Aqueous batteries use water as the solvent for…

First-ever combined heart pump and pig kidney transplant
…gives new hope to patient with terminal illness. Surgeons at NYU Langone Health performed the first-ever combined mechanical heart pump and gene-edited pig kidney transplant surgery in a 54-year-old woman…

Biophysics: Testing how well biomarkers work
LMU researchers have developed a method to determine how reliably target proteins can be labeled using super-resolution fluorescence microscopy. Modern microscopy techniques make it possible to examine the inner workings…





















