New ovarian cancer hope for women

Scientists bring early detection closer
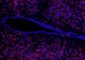
Scientists at the Pacific Northwest Research Institute (PNRI) in Seattle announced a new biomarker for ovarian cancer today. Their discovery promises improved diagnosis of the disease, which usually remains hidden until it is too late for effective treatment.
In the July 1 issue of Cancer Research, the researchers describe a molecule, HE4, associated with ovarian cancer cells. Because the molecule is secreted readily into the blood, its presence should be detectable when simple and inexpensive clinical blood tests are developed.
“Many cancers have a high cure rate if diagnosed early,” Dr. Ingegerd Hellstrom, a principal scientist at PNRI and the lead author of the new paper, says. “But not if diagnosed late. Unfortunately, ovarian carcinoma is most often diagnosed when it is already in an advanced stage. Even after surgery and chemotherapy, relapses are common.”
According to the American Cancer Society, the survival statistics are dismal. Three out of four cases of ovarian cancer are diagnosed in late stages. Last year alone, nearly 14,000 women died of the disease.
The best currently available diagnostic test for ovarian cancer is CA125. It is useful in diagnosing late stage cancers, and in detecting the recurrence of tumors after chemotherapy and radiation. But it is not very effective in identifying early stage disease. It also sometimes indicates the presence of ovarian cancer where there is none. Such “false positive” results lead to dangerous, expensive, and unnecessary treatment.
In the Cancer Research study, the new biomarker, HE4, proved to be at least as effective as CA125. And where no false positive results occurred, HE4’s sensitivity to ovarian carcinoma was 40% higher than that of CA125.
“I’m very anxious to do something for patients,” Hellstrom says. But there is much work to be done, laboratory studies with larger numbers of serum samples, and commercial development to design effective clinical applications of the research. The possibility that a simple and inexpensive blood test can be developed for clinical use is already being studied in a licensing agreement with Fujirebio Diagnostics Incorporated, the creator of CA125.
“Still, this is a step in the right direction,” Hellstrom says. “And we are working as passionately and creatively as we can, to curtail this terrible disease.”
Media Contact
More Information:
http://www.pnri.org/All latest news from the category: Health and Medicine
This subject area encompasses research and studies in the field of human medicine.
Among the wide-ranging list of topics covered here are anesthesiology, anatomy, surgery, human genetics, hygiene and environmental medicine, internal medicine, neurology, pharmacology, physiology, urology and dental medicine.
Newest articles

Webb captures top of iconic horsehead nebula in unprecedented detail
NASA’s James Webb Space Telescope has captured the sharpest infrared images to date of a zoomed-in portion of one of the most distinctive objects in our skies, the Horsehead Nebula….
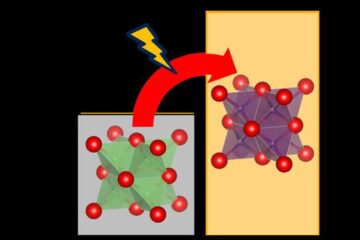
Cost-effective, high-capacity, and cyclable lithium-ion battery cathodes
Charge-recharge cycling of lithium-superrich iron oxide, a cost-effective and high-capacity cathode for new-generation lithium-ion batteries, can be greatly improved by doping with readily available mineral elements. The energy capacity and…
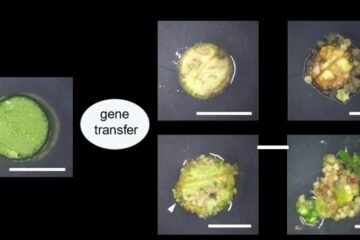
Novel genetic plant regeneration approach
…without the application of phytohormones. Researchers develop a novel plant regeneration approach by modulating the expression of genes that control plant cell differentiation. For ages now, plants have been the…