Lithium’s narrow paths limit batteries
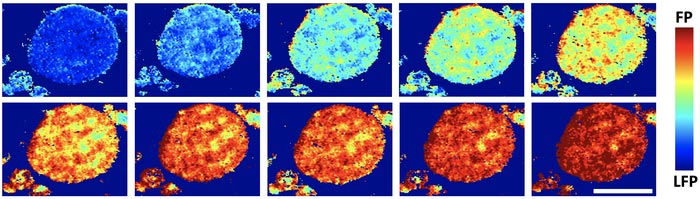
A phase map of an agglomerated particle in a common lithium iron phosphate (LFP) battery electrode shows the charge distribution as it goes from 4% to 86%. FP refers to iron phosphate. Rice University scientists found that the FP phase spreads nonuniformly on an aggregate surface upon charging, rather than the expected even spread of lithium over the surface. The scale bar is 10 microns.
Credit: Mesoscale Materials Science Group/Rice University
Rice study suggests stress among misaligned particles in typical cathodes limits flow.
If you could shrink enough for a fantastic voyage across a lithium battery electrode, you’d see the level of charge at every scale is highly uneven.
This is not good for the battery’s health. Rice University researchers who recognize the problem worked with the Department of Energy to view in great detail how the various particles in an electrode interact with lithium during use.
Specifically, the Rice lab of materials scientist Ming Tang analyzed nano- and micro-scale interactions within lithium iron phosphate cathodes through modeling and imaging offered by the transmission X-ray microscopy capabilities at Brookhaven National Laboratory and Argonne National Laboratory.
Their paper in the American Chemical Society journal ACS Energy Letters supports theories Tang and his colleagues formed several years ago that foresaw how lithium travels in the dynamic environment inside a typical commercial cathode.
Being able to watch sealed cathodes charge and discharge at Brookhaven offered absolute proof.
“Batteries have a lot of particle aggregates that soak up and give up lithium, and we wanted to know what happens on their surfaces, how uniform the reaction is,” said Tang, an associate professor of materials science and nanoengineering. “In general, we always want a more uniform reaction so we can charge the battery faster.”
In images taken at Brookhaven’s powerful X-ray synchrotron, the researchers saw some regions inside the cathode were better at absorption than others. The ability to look at single or aggregated particles in 3D showed that rather than reacting over their entire surfaces, lithium favored particular regions over others.
“This is very different from conventional wisdom,” Tang said. “The most interesting observation is that these reaction regions are shaped like one-dimensional filaments lying across the surface of these aggregated particles. It was kind of weird, but it matched what we saw in our models.”
Tang said the lithium filaments looked something like thick nanotubes and were several hundred nanometers wide and several microns long.
He said stress between misaligned crystallites in the particle agglomerates prevents lithium from being uniformly inserted into or extracted from the aggregate surface because that will generate too large an energy penalty. Instead, lithium is forced to flow into or out of the aggregates at “hot spots” that develop the filament shape.
What does this mean for battery performance?
“This is a bad thing,” Tang said. “Because the lithium can’t go into the cathode uniformly, it slows down the intercalation mechanics.
“What our study offers is some potential ways to help make lithium insertion or extraction more uniform on these aggregates or individual particles,” he said. “Introducing some porosity in the particle agglomerates might sacrifice some energy density, but at the same time would allow lithium to go in more uniformly. That could allow you to get more energy at a given charge/discharge rate.
“Another thought is if we can somehow align the orientation of these small particles so their maximum expansion is perpendicular to each other, they’ll better accommodate lithium intercalation,” he said.
That would be a challenge for battery manufacturers, he admitted.
“We don’t have enough experience in synthesis to know how to make that happen,” Tang said. “What we’re providing is bait. Let’s see if somebody bites.”
Rice graduate alumni Fan Wang and Kaiqi Yang are co-lead authors of the paper. Co-authors are Mingyuan Ge, Jiajun Wang, Jun Wang, Xianghi Xiao and Wah-Keat Lee, all of Brookhaven National Laboratory, Upton, New York; and Linsen Li of Shanghai Jiao Tong University.
The Department of Energy, Basic Energy Sciences (DE-SC0019111) and the National Science Foundation (CMMI-1929949) supported the research.
Read the abstract at https://pubs.acs.org/doi/10.1021/acsenergylett.2c00226.
This news release can be found online at https://news.rice.edu/news/2022/lithiums-narrow-paths-limit-batteries.
Follow Rice News and Media Relations via Twitter @RiceUNews.
Related materials:
Mesoscale Materials Science Group (Tang lab): http://tanggroup.rice.edu/research/
Materials Science and NanoEngineering: https://msne.rice.edu
George R. Brown School of Engineering: https://engineering.rice.edu
Images for download:
https://news-network.rice.edu/news/files/2022/04/0418_BATTERY-1-WEB.jpg
A phase map of an agglomerated particle in a common lithium iron phosphate (LFP) battery electrode shows the charge distribution as it goes from 4% to 86%. FP refers to iron phosphate. Rice University scientists found that the FP phase spreads nonuniformly on an aggregate surface upon charging, rather than the expected even spread of lithium over the surface. The scale bar is 10 microns. (Credit: Mesoscale Materials Science Group/Rice University)
https://news-network.rice.edu/news/files/2022/04/0418_BATTERY-2-WEB.jpg
A study by Rice University materials scientists suggests that lithium batteries would benefit from more porous secondary (agglomerated) particles with better-aligned crystallites that don’t limit lithium distribution. The scientists studied 3D transmission X-ray images of cycled battery electrodes to analyze the phase change between lithium iron phosphate (blue) and iron phosphate (red) on the surface of particle agglomerates that make up the electrodes. (Credit: Mesoscale Materials Science Group/Rice University)
https://news-network.rice.edu/news/files/2022/04/0418_BATTERY-ANIM-1.gif
https://news-network.rice.edu/news/files/2022/04/0418_BATTERY-ANIM-2.gif
Animated GIFs of a phase-mapped secondary (aggregated) particle in a lithium iron phosphate cathode. (Credit: Mesoscale Materials Science Group/Rice University)
Located on a 300-acre forested campus in Houston, Rice University is consistently ranked among the nation’s top 20 universities by U.S. News & World Report. Rice has highly respected schools of Architecture, Business, Continuing Studies, Engineering, Humanities, Music, Natural Sciences and Social Sciences and is home to the Baker Institute for Public Policy. With 4,052 undergraduates and 3,484 graduate students, Rice’s undergraduate student-to-faculty ratio is just under 6-to-1. Its residential college system builds close-knit communities and lifelong friendships, just one reason why Rice is ranked No. 1 for lots of race/class interaction and No. 1 for quality of life by the Princeton Review. Rice is also rated as a best value among private universities by Kiplinger’s Personal Finance.
Journal: ACS Energy Letters
DOI: 10.1021/acsenergylett.2c00226
Method of Research: Experimental study
Subject of Research: Not applicable
Article Title: Reaction Heterogeneity in LiFePO4 Agglomerates and the Role of Intercalation-Induced Stress
Article Publication Date: 11-Apr-2022
COI Statement: None
Media Contacts
Mike Williams
Rice University
mikewilliams@rice.edu
Office: 713-348-6728
Jeff Falk
Rice University
jfalk@rice.edu
Office: 713-348-6775
All latest news from the category: Materials Sciences
Materials management deals with the research, development, manufacturing and processing of raw and industrial materials. Key aspects here are biological and medical issues, which play an increasingly important role in this field.
innovations-report offers in-depth articles related to the development and application of materials and the structure and properties of new materials.
Newest articles

Combining robotics and ChatGPT
TUM professor uses ChatGPT for choreographies with flying robots. Prof. Angela Schoellig has proved that large language models can be used safely in robotics. ChatGPT develops choreographies for up to…

How the Immune System Learns from Harmless Particles
Our lungs are bombarded by all manner of different particles every single day. Whilst some are perfectly safe for us, others—known as pathogens—have the potential to make us ill. The…

Biomarkers identified for successful treatment of bone marrow tumours
CAR T cell therapy has proven effective in treating various haematological cancers. However, not all patients respond equally well to treatment. In a recent clinical study, researchers from the University…





















