Researchers identify tube-forming proteins in mycobacterial outer envelope
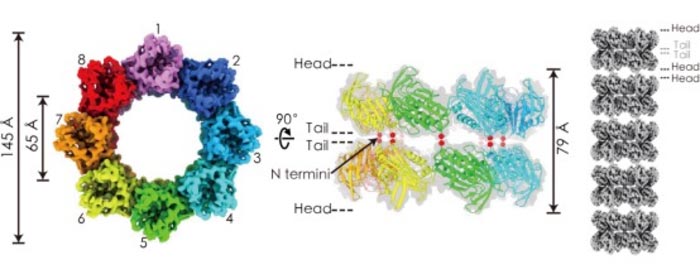
Cryo-EM density map of amphipol-solubilized mtTiME-FL reconstructed by D8 symmetry in top view (left) and side view (middle). Tube model of TiME stacked by double-layered complexes (right).
Credit: CAI Xiaoying et al.
The research team led by Prof. GONG Weimin from the University of Science and Technology of China (USTC), collaborating with Dr. ZHOU Zhenghong from the University of California, Los Angeles (UCLA), clarified the architecture of tube-forming proteins that function as a protein transport tube across the mycobacterial outer envelope. The study was published in Science Advances.
Tuberculosis-causing mycobacteria have been plaguing human society as the leading cause of death by a single infectious agent. Due to the distinctive structure of a thick cell wall and complex capsule layers, mycobacteria rely on a specialized protein secretion system. However, these secretion channels embedded within the mycobacterial cell envelope have not been reported.
In this study, the researchers found that Mycobacterium tuberculosis Rv3705c and its homologous MSMEG_6251 in Mycobacterium smegmatis (M. smegmatis) were tube-forming proteins in the mycobacterial envelope (TiME, referred to as mtTiME and msTiME respectively).
By combining x-ray crystallography and cryo-electron microscopy (cryo-EM), they revealed that both proteins formed rotationally single-layered rings of ~6 nm inner diameter with eightfold (C8) symmetry, and two layers of TiME rings packed together in a tail-to-tail manner into a ring-shaped complex, which, in turn, stacked together in a head-to-head manner to form tubes.
In addition, in vivo subcellular localization results showed that TiME distributed on the cell envelope of M. smegmatis, with higher abundance in the capsule and cell wall, and knocking out the TiME gene significantly decreased the amount of secreted proteins whose molecular weight was below ~22 kDa in the Δmstime culture medium.
Comparing the protein compositions in wild-type and knocked-out strain after disrupting the capsule and surface of the cell wall, the researchers found no notable difference in the amount of proteins, which indicated that TiME formed protein transport channels across the mycobacterial outer envelope.
Cryo-EM density map of amphipol-solubilized mtTiME-FL reconstructed by D8 symmetry in top view (left) and side view (middle). Tube model of TiME stacked by double-layered complexes (right). (Image by CAI Xiaoying et al. )
The identification and analysis of tube-forming proteins in M. smegmatis suggested that TiME is an enabler in protein secretion in mycobacteria, providing a novel method for studying the substance transport mechanism of pathogenic mycobacteria and structure-based inhibitor design.
Journal: Science Advances
DOI: 10.1126/sciadv.abg5656
Article Title: Identification and architecture of a putative secretion tube across mycobacterial outer envelope
Media Contact
Jane fan
University of Science and Technology of China
qfan@ustc.edu.cn
All latest news from the category: Life Sciences and Chemistry
Articles and reports from the Life Sciences and chemistry area deal with applied and basic research into modern biology, chemistry and human medicine.
Valuable information can be found on a range of life sciences fields including bacteriology, biochemistry, bionics, bioinformatics, biophysics, biotechnology, genetics, geobotany, human biology, marine biology, microbiology, molecular biology, cellular biology, zoology, bioinorganic chemistry, microchemistry and environmental chemistry.
Newest articles

Microscopic basis of a new form of quantum magnetism
Not all magnets are the same. When we think of magnetism, we often think of magnets that stick to a refrigerator’s door. For these types of magnets, the electronic interactions…

An epigenome editing toolkit to dissect the mechanisms of gene regulation
A study from the Hackett group at EMBL Rome led to the development of a powerful epigenetic editing technology, which unlocks the ability to precisely program chromatin modifications. Understanding how…

NASA selects UF mission to better track the Earth’s water and ice
NASA has selected a team of University of Florida aerospace engineers to pursue a groundbreaking $12 million mission aimed at improving the way we track changes in Earth’s structures, such…





















