Researchers can customize cavities in new porous material
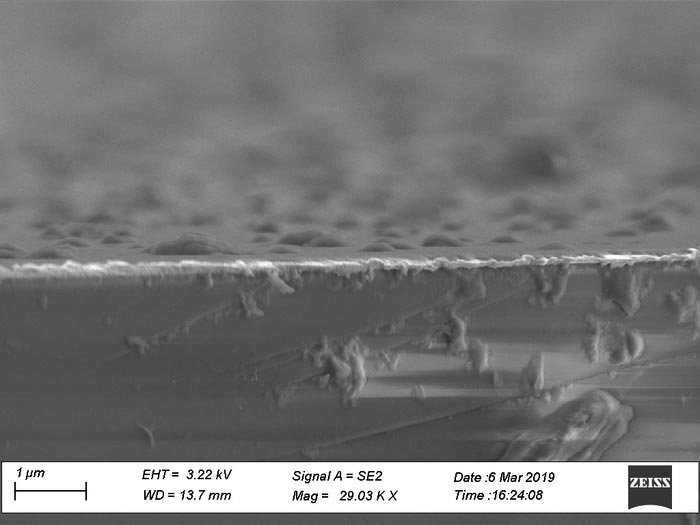
Image of the porous material in cross-section enlarged 29,000 times. The white line is the gold plate on which the material is built up.
Credit: Martin Ratsch
Researchers at the University of Gothenburg have produced a porous and stable material in which the cavities can be used to store various substances. The material can be used both in the pharmaceutical industry and for filtration at the molecular level.
Porous materials are very interesting to scientists since they can interact with guest particles, such as ions, atoms, and molecules, in the cavities of the material. Inorganic porous materials like zeolites are already being used in detergents, where they play a role by exchanging their own sodium ions for the calcium ions in water to turn it into soft water.
The problem with inorganic porous materials is the lack of flexibility in designing them as needed.
Easier to design
“We based our discovery on research into covalent carbon bonds awarded the Nobel Prize in 2010. In the past, we have been able to create organic, porous materials in powder form using carbon molecules, but this approach is best suited to storing gases in the cavities. Now we can build a material with layers of thin films that is much more stable and easier to design to our specifications,” says Martin Ratsch, a doctoral student in the Department of Chemistry and Molecular Biology.
Ratsch builds porous films on a thin plate of gold, where covalent carbon bonds create a stable porous surface. By adding a controlled amount of carbon-based molecules activated by the element palladium, he can create porous films with the desired thickness and with cavities of the right size. The result is a smooth surface that allows researchers to combine layers of films with different properties, a bit like a chocolate biscuit. Substances can be placed in the cavities inside the material as required.
“There are several fields of application. The porous film can also act as a membrane in batteries, when you want to control the number of ions that can pass through.”
Works also as a filter
Ratsch also points out that the new material could be an interesting way of filtering solutions down to the molecular level, since the size of the cavities can be determined. It could theoretically be possible to filter vodka so that the ethanol molecules become trapped, while the smaller water molecules can pass through the cavities.
The porous film has been produced in the laboratory on a very small scale. Much research remains to be done, especially if the process is to be less expensive.
“We use a thin sheet of gold to construct the material and palladium as a catalyst to create the covalent carbon bonds. We need to find another substance as a catalyst before we can scale up production. That should be the next step in the research, and it may take 10 years before this material becomes commercially viable.”
Facts at a glance: Covalent carbon bonds
A covalent bond, or electron pair bond, occurs when two or more atoms share one, two, or three electron pairs among them. Classical atomic physics describes this as filling the outermost electron shell. Covalent bonds create stable molecular chains that researchers use to build new materials with high regularity.
Organic molecules always contain carbon and hydrogen, but since the carbon atom is relatively poor in electrons, it needs help to form covalent bonds. Research has shown that the element palladium serves as a catalyst that helps carbon form covalent bonds. The process is unique because it can be carried out under mild conditions and with high precision. This has been crucial in producing the porous film built up using various carbon compounds.
The dissertation’s title: Towards 3D Covalent Organic Framework Films
Contact: Martin Ratsch, doctoral student at the Department of Chemistry and Molecular Biology at the University of Gothenburg. Phone: +46 (0)766-22 91 01, email: martin.ratsch@chem.gu.se
All latest news from the category: Life Sciences and Chemistry
Articles and reports from the Life Sciences and chemistry area deal with applied and basic research into modern biology, chemistry and human medicine.
Valuable information can be found on a range of life sciences fields including bacteriology, biochemistry, bionics, bioinformatics, biophysics, biotechnology, genetics, geobotany, human biology, marine biology, microbiology, molecular biology, cellular biology, zoology, bioinorganic chemistry, microchemistry and environmental chemistry.
Newest articles

Why getting in touch with our ‘gerbil brain’ could help machines listen better
Macquarie University researchers have debunked a 75-year-old theory about how humans determine where sounds are coming from, and it could unlock the secret to creating a next generation of more…

Attosecond core-level spectroscopy reveals real-time molecular dynamics
Chemical reactions are complex mechanisms. Many different dynamical processes are involved, affecting both the electrons and the nucleus of the present atoms. Very often the strongly coupled electron and nuclear…

Free-forming organelles help plants adapt to climate change
Scientists uncover how plants “see” shades of light, temperature. Plants’ ability to sense light and temperature, and their ability to adapt to climate change, hinges on free-forming structures in their…





















