Nanomaterials mimicking natural enzymes with superior catalytic activity and selectivity
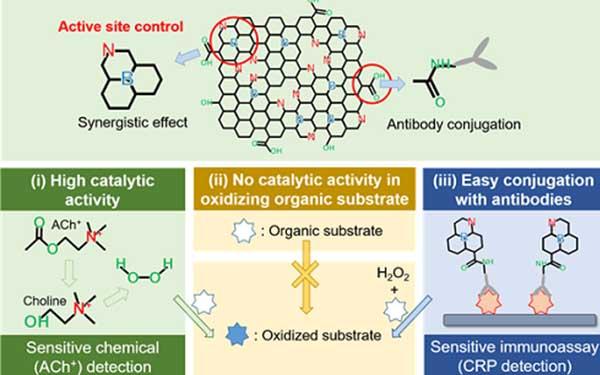
This is a schematic illustration of NB-rGO Reactions in Bioassays. Credit: KAIST
Enzymes are the main catalysts in our body and are widely used in bioassays. In particular, peroxidase, which oxidizes transparent colorimetric substrates to become a colored product in the presence of hydrogen peroxide, is the most common enzyme that is used in colorimetric bioassays.
However, natural enzymes consisting of proteins are unstable against temperature and pH, hard to synthesize, and costly. Nanozymes, on the other hand, do not consist of proteins, meaning the disadvantages of enzymes can be overcome with their robustness and high productivity.
In contrast, most nanonzymes do not have selectivity; for example, peroxidase-mimicking nanozymes demonstrate oxidase-like activity that oxidizes colorimetric substrates in the absence of hydrogen peroxide, which keeps them away from precisely detecting the target materials, such as hydrogen peroxide.
Professor Jinwoo Lee from the Department of Chemical and Biomolecular Engineering and his team were able to synthesize a peroxidase-mimicking nanozyme with superior catalytic activity and selectivity toward hydrogen peroxide.
Co-doping of nitrogen and boron into graphene, which has negligible peroxidase-like activity, selectively increased the peroxidase-like activity without oxidase-like activity to accurately mimic the nature peroxidase and has become a powerful candidate to replace the peroxidase.
The experimental results were also verified with computational chemistry. The nitrogen and boron co-doped graphene was also applied to the colorimetric detection of acetylcholine, which is an important neurotransmitter and successfully detected the acetylcholine even better than the nature peroxidase.
Professor Lee said, “We began to study nanozymes due to their potential for replacing existing enzymes. Through this study, we have secured core technologies to synthesize nanozymes that have high enzyme activity along with selectivity. We believe that they can be applied to effectively detect acetylcholine for quickly diagnosing Alzheimer's disease.
###
This research, led by PhD Min Su Kim, was published in ACS Nano (10.1021/acsnano.8b09519) on March 25, 2019.
Media Contact
All latest news from the category: Life Sciences and Chemistry
Articles and reports from the Life Sciences and chemistry area deal with applied and basic research into modern biology, chemistry and human medicine.
Valuable information can be found on a range of life sciences fields including bacteriology, biochemistry, bionics, bioinformatics, biophysics, biotechnology, genetics, geobotany, human biology, marine biology, microbiology, molecular biology, cellular biology, zoology, bioinorganic chemistry, microchemistry and environmental chemistry.
Newest articles

Sea slugs inspire highly stretchable biomedical sensor
USC Viterbi School of Engineering researcher Hangbo Zhao presents findings on highly stretchable and customizable microneedles for application in fields including neuroscience, tissue engineering, and wearable bioelectronics. The revolution in…

Twisting and binding matter waves with photons in a cavity
Precisely measuring the energy states of individual atoms has been a historical challenge for physicists due to atomic recoil. When an atom interacts with a photon, the atom “recoils” in…
Nanotubes, nanoparticles, and antibodies detect tiny amounts of fentanyl
New sensor is six orders of magnitude more sensitive than the next best thing. A research team at Pitt led by Alexander Star, a chemistry professor in the Kenneth P. Dietrich…





















