How a single protein could unlock age-related vision loss
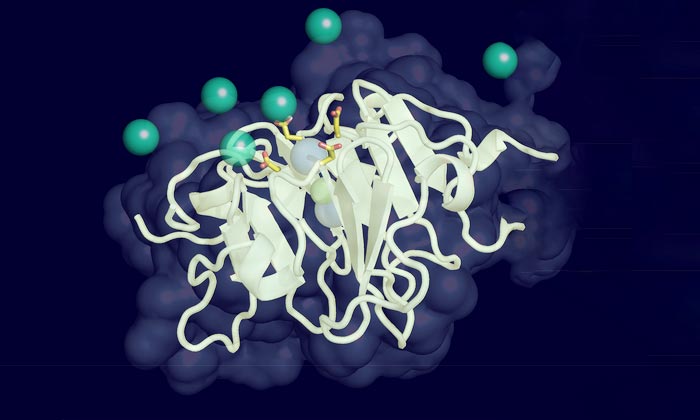
Vitronectin illustration
Credit: Sanford Burnham Prebys/Francesca Marassi
Research led by Sanford Burnham Prebys professor Francesca Marassi, Ph.D., is helping to reveal the molecular secrets of macular degeneration, which causes almost 90% of all age-related vision loss. The study, published recently in the Biophysical Journal, describes the flexible structure of a key blood protein involved in macular degeneration and other age-related diseases, such as Alzheimer’s and atherosclerosis.
“Proteins in the blood are under constant and changing pressure because of the different ways blood flows throughout the body,” says Marassi. “For example, blood flows more slowly through small blood vessels in the eyes compared to larger arteries around the heart. Blood proteins need to be able to respond to these changes, and this study gives us fundamental truths about how they adapt to their environment, which is critical to targeting those proteins for future treatments.”
There are hundreds of proteins in our blood, but the researchers focused on vitronectin, one of the most abundant. In addition to circulating in high concentrations in the blood, vitronectin is found in the scaffolding between cells and is also an important component of cholesterol.
Vitronectin is a key player in many age-related diseases, but for Marassi’s team, the most promising target is macular degeneration, which affects as many as 11 million people in the United States. This number is expected to double by 2050.
“This protein is an important target for macular degeneration because it accumulates in the back of the eye, causing vision loss. Similar deposits appear in the brain in Alzheimer’s disease and in the arteries in atherosclerosis,” says Marassi. “We want to understand why this happens and leverage this knowledge to develop new treatments.”
To approach this question, the researchers were interested in learning how the protein changes its structure at different temperatures and under different levels of pressure, approximating what happens in the human body.
“Determining the structure of a protein is the most important part of determining its function,” adds Marassi.
Through detailed biochemical analysis, the researchers found that the protein can subtly change its shape under pressure. These changes cause it to bond more easily to calcium ions in the blood, which the researchers suggest leads to the buildup of calcified plaque deposits characteristic of macular degeneration and other age-related diseases.
“It’s a very subtle rearrangement of the molecular structure, but it has a big impact on how the protein functions,” says Marassi. “The more we learn about the protein on a structural and mechanistic level, the better chance we have of successfully targeting it with treatments.”
These structural insights will streamline the development of treatments for macular degeneration because it will allow researchers and their partners in the biotech industry to custom-design antibodies that selectively block the protein’s calcium binding without disrupting its other important functions in the body.
“It will take some time to convert it into a clinical treatment, but we hope to have a working antibody as a potential treatment in a few years’ time,” says Marassi. “And since this protein is so abundant in the blood, there may be other exciting applications for this new knowledge that we don’t even know about yet.”
Additional authors on the study include Ye Tian, Ph.D., Kyungsoo Shin, Ph.D., Alexander E. Aleshin, Ph.D., Sanford Burnham Prebys Medical Discovery Institute and Wonpil Im, Lehigh University.
About Sanford Burnham Prebys
Sanford Burnham Prebys is a preeminent, independent biomedical research institute dedicated to understanding human biology and disease and advancing scientific discoveries to profoundly impact human health. For more than 45 years, our research has produced breakthroughs in cancer, neuroscience, immunology and children’s diseases, and is anchored by our NCI-designated Cancer Center and advanced drug discovery capabilities. For more information, visit us at SBPdiscovery.org or on Facebook facebook.com/SBPdiscovery and on Twitter @SBPdiscovery.
Journal: Biophysical Journal
DOI: 10.1016/j.bpj.2022.08.044
Article Title: Calcium-induced environmental adaptability of the blood protein vitronectin
Article Publication Date: 1-Sep-2022
Media Contact
Miles Martin
Sanford Burnham Prebys
mmartin@sbpdiscovery.org
All latest news from the category: Life Sciences and Chemistry
Articles and reports from the Life Sciences and chemistry area deal with applied and basic research into modern biology, chemistry and human medicine.
Valuable information can be found on a range of life sciences fields including bacteriology, biochemistry, bionics, bioinformatics, biophysics, biotechnology, genetics, geobotany, human biology, marine biology, microbiology, molecular biology, cellular biology, zoology, bioinorganic chemistry, microchemistry and environmental chemistry.
Newest articles

Why getting in touch with our ‘gerbil brain’ could help machines listen better
Macquarie University researchers have debunked a 75-year-old theory about how humans determine where sounds are coming from, and it could unlock the secret to creating a next generation of more…

Attosecond core-level spectroscopy reveals real-time molecular dynamics
Chemical reactions are complex mechanisms. Many different dynamical processes are involved, affecting both the electrons and the nucleus of the present atoms. Very often the strongly coupled electron and nuclear…

Free-forming organelles help plants adapt to climate change
Scientists uncover how plants “see” shades of light, temperature. Plants’ ability to sense light and temperature, and their ability to adapt to climate change, hinges on free-forming structures in their…





















