First observation of how water molecules move near a metal electrode
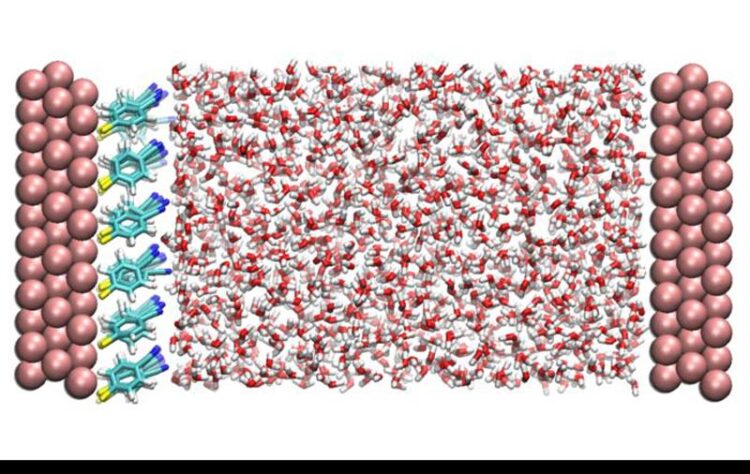
A snapshot taken from the computer simulation model of the system in this study. On both sides are three layers of gold atoms representing the metal electrode with adsorbed organic molecules on the left electrode. The space between the electrodes is filled with water molecules.
Credit: Institute for Basic Science
Movements of water molecules change depending on the applied voltage.
A collaborative team of experimental and computational physical chemists from South Korea and the United States have made an important discovery in the field of electrochemistry, shedding light on the movement of water molecules near metal electrodes. This research holds profound implications for the advancement of next-generation batteries utilizing aqueous electrolytes.
In the nanoscale realm, chemists typically utilize laser light to illuminate molecules and measure spectroscopic properties to visualize molecules. However, studying the behavior of water molecules near metal electrodes proved challenging due to the overwhelming interference from metal atoms in the electrode itself. Additionally, water molecules distant from the electrode surface also contribute to the response of the applied light, complicating the selective observation of molecules at the liquid-metal electrode interface.
Led by Professor Martin Zanni from the University of Wisconsin at Madison and Director CHO Minhaeng from the Center for Molecular Spectroscopy and Dynamics within the Institute for Basic Science (IBS) addressed this challenge with newly developed spectroscopic techniques coupled with computer simulations. In order to minimize the interference from the metals, the authors coated the surface of the electrode with specially designed organic molecules. Then, surface-enhanced femtosecond (10-15 second) two-dimensional vibrational spectroscopy was employed to observe the changes in the movement of water molecules near the metal electrode.
Depending on the magnitude and polarity of the applied voltage on the metal electrode, the researchers observed, for the first time, either a deceleration or acceleration of the motion of water molecules near the electrode. “When a positive voltage is applied to the electrode, the movement of nearby water molecules slows down. Conversely, when a negative voltage is applied, the opposite is observed both in femtosecond vibrational spectroscopy and in computer simulations,” explains Dr. Kwac.
“The results of this study provide crucial information for understanding electrochemical reactions, offering essential physical insights necessary for the research and development of aqueous electrolyte batteries in the future,” comments Director CHO Minhaeng of the IBS Center for Molecular Spectroscopy and Dynamics, a corresponding author of the study.
This outcome implies a close relationship between electrochemical reactions involving water on the surface of electrodes and the dynamics of interfacial water molecules. It is expected to not only advance our understanding of fundamental electrochemical processes but also pave the way for the design of more efficient and sustainable battery technologies.
This research was published in the Proceedings of the National Academy of Sciences (PNAS) on December 18, 2023.
Journal: Proceedings of the National Academy of Sciences
Method of Research: Experimental study
Subject of Research: Not applicable
Article Title: The hydrogen-bonding dynamics of water to a nitrile functionalized electrode is modulated by voltage according to 2D IR spectroscopy
Article Publication Date: 18-Dec-2023
Media Contact
William Suh
Institute for Basic Science
willisuh@ibs.re.kr
Office: 82-010-379-37830
All latest news from the category: Life Sciences and Chemistry
Articles and reports from the Life Sciences and chemistry area deal with applied and basic research into modern biology, chemistry and human medicine.
Valuable information can be found on a range of life sciences fields including bacteriology, biochemistry, bionics, bioinformatics, biophysics, biotechnology, genetics, geobotany, human biology, marine biology, microbiology, molecular biology, cellular biology, zoology, bioinorganic chemistry, microchemistry and environmental chemistry.
Newest articles

Microscopic basis of a new form of quantum magnetism
Not all magnets are the same. When we think of magnetism, we often think of magnets that stick to a refrigerator’s door. For these types of magnets, the electronic interactions…

An epigenome editing toolkit to dissect the mechanisms of gene regulation
A study from the Hackett group at EMBL Rome led to the development of a powerful epigenetic editing technology, which unlocks the ability to precisely program chromatin modifications. Understanding how…

NASA selects UF mission to better track the Earth’s water and ice
NASA has selected a team of University of Florida aerospace engineers to pursue a groundbreaking $12 million mission aimed at improving the way we track changes in Earth’s structures, such…





















