Deconstructing the mechanics of bone marrow disease
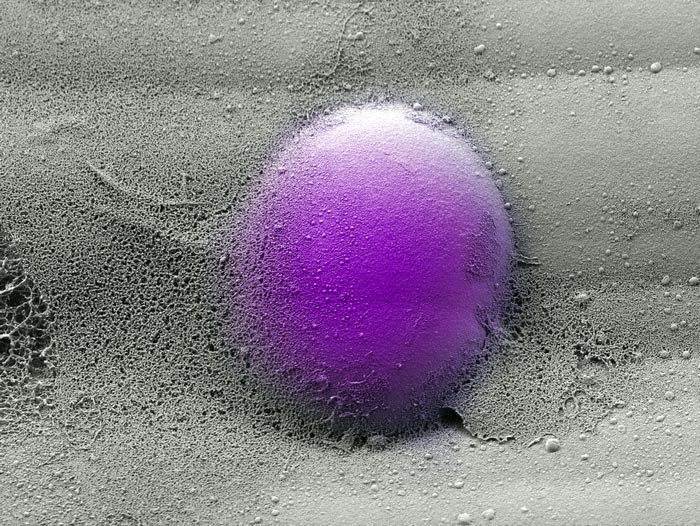
The team developed an alginate-based hydrogel system that mimics the viscoelasticity of the natural extracellular matrix in bone marrow. By tweaking the balance between elastic and viscous properties in these artificial ECMs, they could recapitulate the viscoelasticity of healthy and scarred fibrotic bone marrow, and study the effects on human monocytes placed into these artificial ECMs.
Credit: Wyss Institute at Harvard University
New understanding of how mechanical features of bone marrow affect resident immune cells in fibrotic cancer points to future therapeutic strategies for cancers and fibrotic diseases.
By Benjamin Boettner
Fibrosis is the thickening of various tissues caused by the deposition of fibrillar extracellular matrix (ECM) in tissues and organs as part of the body’s wound healing response to various forms of damage. When accompanied by chronic inflammation, fibrosis can go into over-drive and produce excess scar tissue that cannot be degraded anymore. This process causes many diseases in multiple organs, including lung fibrosis induced by smoking or asbestos, liver fibrosis induced by alcohol abuse, and heart fibrosis often following heart attacks. Fibrosis can also occur in the bone marrow (BM), the spongy tissue inside some of our bones that houses blood-producing hematopoietic stem cells (HSCs), which leads to scarring and the disruption of normal functions.
One example are chronic blood cancers known as “myeloproliferative neoplasms” (MPNs), in which patients can develop fibrotic BM (myelofibrosis) that disrupts the normal production of blood cells. Monocytes, a type of white blood cell belonging to the group of myeloid cells, are overproduced from HSCs in MPN, and contribute to the inflammation in the BM environment (niche). However, how the fibrotic bone marrow niche itself impacts the function of monocytes and inflammation in the bone marrow was unknown.
Now, a collaborative team at Harvard’s Wyss Institute for Biologically Inspired Engineering at Harvard University, John A. Paulson School of Engineering and Applied Sciences (SEAS), Dana-Farber Cancer Institute (DFCI), and Brigham and Women’s Hospital (Brigham), has created a programmable hydrogel-based in vitro model mimicking healthy and fibrotic human BM. Combining this system with mouse in vivo models of myelofibrosis (MF), they demonstrated that monocytes decide whether to enter a pro-inflammatory state and go on to differentiate into inflammatory dendritic cells based on specific mechanical properties of the BM niche with its densely packed ECM molecules. Importantly, the team found that a drug inhibiting the PI3K-gamma protein toned down these pathological mechanical effects on monocytes, and reduced their numbers as well as the numbers of inflammatory myeloid cells in mice with MF. The findings are published in Nature Materials.
“Our study shows that the differentiation state of monocytes, which are key players in the immune system, is highly regulated by mechanical changes in the ECM they encounter. Specifically, the ECM’s viscoelasticity has been a historically under-appreciated aspect of its mechanical properties that we find correlates strongly between our in vitro and the in vivo models and human disease,” said Wyss Core Faculty member David Mooney, Ph.D., who co-led the study with DFCI researcher Kai Wucherpfennig, M.D., Ph.D. “It turns out that MF is a mechano-related disease that could be treated by interfering with the mechanical signaling in bone marrow cells,” Mooney added.
Mooney is also the Robert P. Pinkas Family Professor of Bioengineering at SEAS, and leads the Wyss Institute’s Immuno-Materials Platform. Wucherpfennig is Director of DFCI’s Center for Cancer Immunotherapy Research, Professor of Neurobiology at Brigham and Harvard Medical School, and an Associate Member of the Broad Institute of MIT and Harvard. Mooney, together with co-senior author F. Stephen Hodi, M.D. also heads the Immuno-engineering to Improve Immunotherapy (i3) Center, which aims to create new biomaterials-based approaches to enhance immune responses against tumors. Hodi is Director of the Melanoma Center and The Center for Immuno-Oncology at DFCI, and a Professor of Medicine at Harvard Medical School (HMS).
Gleaning mechanical bone marrow failure
The mechanical properties of most biological materials are determined by their “viscoelastic” characteristics. Unlike purely elastic substances likea vibrating quartz, which store elastic energy when mechanically stressed and quickly recover to their original state once the stress is removed (slow-relaxing), viscoelastic substances also have a viscous component. Like the viscosity of honey, this allows them to dissipate stress under mechanical strain by rapid stress relaxation. Viscous materials are thus fast-relaxing materials in contrast to slow-relaxing purely elastic materials.
The team developed an alginate-based hydrogel system that mimics the viscoelasticity of natural ECM which allowed them to tune the elasticity independent from other physical and biochemical properties. By tweaking the balance between elastic and viscous properties in these artificial ECMs, they could recapitulate the viscoelasticity of healthy and scarred fibrotic BM, whose elasticity is increased by excess ECM fibers. Human monocytes placed into these artificial ECMs constantly push and pull at them and in turn respond to the materials’ mechanical characteristics.
“We found that stiff and more elastic slow-relaxing artificial ECMs induced immature monocytes to differentiate into monocytes with a pro-inflammatory program strongly resembling that of monocytes in MF patients, and the monocytes to differentiate further into inflammatory dendritic cells. More viscous fast-relaxing artificial ECMs suppressed this MF-like effect on monocytes,” said co-first author Kyle Vining, Ph.D., who worked as a Postdoctoral Fellow on Mooney’s team. “This opened up the possibility of a mechanical checkpoint that could be disrupted in myelofibrotic bone marrow, and also may be at play in other fibrotic diseases.” Vinning is now Assistant Professor of Preventive and Restorative Sciences at the University of Pennsylvania.
Next, the team investigated how the mechanical characteristics of stiff and elastic hydrogels compared to those in actual BM affected by MF. They took advantage of a mouse model in which an activating mutation in a gene known as Jak2 causes MPN, pro-inflammatory signaling in the BM, and development of MF, similar to the disease process in human patients with MPN. When they investigated the mechanical properties of BM in the animals’ femur bones, using a nanoindentation probe, the researchers measured a higher stiffness than in non-fibrotic BM. “Importantly, we found that the pathologic grading of MF in the animal model was significantly correlated with changes in viscoelasticity,” said co-first author Anna Marneth, Ph.D., who spearheaded the experiments in the mouse model as a Postdoctoral Fellow working with Ann Mullally, M.D., a Principal Investigator at the Brigham and DFCI, and another senior author on the study.
Targeting dysregulated bone marrow mechanics
An important question was whether monocytes’ response to the mechanical impact of the fibrotic BM niche could be therapeutically targeted. The researchers focused on an isoform of the phosphoinositide 3-kinase (PI3K)-gamma protein, which is specifically expressed in monocytes and closely related immune cells. PI3K-gamma is known for regulating the assembly of a cell-stiffening filamentous cytoskeleton below the cell surface that expands in response to mechanical stress, which the team also observed in monocytes encountering a fibrotic ECM. Indeed, when they added a drug that inhibits PI3K-gamma to stiff elastic artificial ECMs, it toned down their pro-inflammatory response and, when given as an oral treatment to MF mice, significantly lowered the number of monocytes and dendritic cells in their BM.
“This research opens new avenues for modifying immune cell function in fibrotic diseases that are currently difficult to treat. The results are also highly relevant to human cancers with a highly fibrotic microenvironment, such as pancreatic cancer,” said Wucherpfennig.
“This study is a terrific advance at the interface between immunology and mechanobiology, which shows how targeting the mechanical environment of a tissue, rather than the genetic underpinnings of the disease, can potentially enable a new generation of powerful therapeutic interventions,” said Wyss Founding Director Donald Ingber, M.D., Ph.D., who is also the Judah Folkman Professor of Vascular Biology at Harvard Medical School and Boston Children’s Hospital, and Hansjörg Wyss Professor of Bioinspired Engineering at the Harvard John A. Paulson School of Engineering and Applied Sciences.
Other authors on the study are Kwasi Adu-Berchie, Joshua M. Grolman, Ph.D.; Christina M. Tringides; Yutong Liu; Waihay J. Wong, M.D., Ph.D.; Olga Pozdnyakova, M.D.; Mariano Severgnini, Ph.D.; Alexander Stafford, and Georg N. Duda, Ph.D. The study was funded by the National Cancer Institute of the National Institutes of Health (Award #U01CA214369), the National Institute of Dental & Craniofacial Research of the National Institutes of Health (Award #K08DE025292 and #K99DE030084), the Federal Drug Administration (Award #R01FD006589), and the Harvard University Materials Research Science and Engineering Center (Grant #DMR 1420570).
PRESS CONTACTS
Wyss Institute for Biologically Inspired Engineering at Harvard University
Benjamin Boettner, benjamin.boettner@wyss.harvard.edu, +1 617-432-8232
The Wyss Institute for Biologically Inspired Engineering at Harvard University (www.wyss.harvard.edu) is a research and development engine for disruptive innovation powered by biologically-inspired engineering with visionary people at its heart. Our mission is to transform healthcare and the environment by developing ground-breaking technologies that emulate the way Nature builds and accelerate their translation into commercial products through formation of startups and corporate partnerships to bring about positive near-term impact in the world. We accomplish this by breaking down the traditional silos of academia and barriers with industry, enabling our world-leading faculty to collaborate creatively across our focus areas of diagnostics, therapeutics, medtech, and sustainability. Our consortium partners encompass the leading academic institutions and hospitals in the Boston area and throughout the world, including Harvard’s Schools of Medicine, Engineering, Arts & Sciences and Design, Beth Israel Deaconess Medical Center, Brigham and Women’s Hospital, Boston Children’s Hospital, Dana–Farber Cancer Institute, Massachusetts General Hospital, the University of Massachusetts Medical School, Spaulding Rehabilitation Hospital, Boston University, Tufts University, Charité – Universitätsmedizin Berlin, University of Zürich, and Massachusetts Institute of Technology.
The Harvard John A. Paulson School of Engineering and Applied Sciences (http://seas.harvard.edu) serves as the connector and integrator of Harvard’s teaching and research efforts in engineering, applied sciences, and technology. Through collaboration with researchers from all parts of Harvard, other universities, and corporate and foundational partners, we bring discovery and innovation directly to bear on improving human life and society.
Media Contact
Benjamin Boettner
Wyss Institute for Biologically Inspired Engineering at Harvard
Benjamin.Boettner@wyss.harvard.edu
Office: 917-913-8051
Media Contact
All latest news from the category: Life Sciences and Chemistry
Articles and reports from the Life Sciences and chemistry area deal with applied and basic research into modern biology, chemistry and human medicine.
Valuable information can be found on a range of life sciences fields including bacteriology, biochemistry, bionics, bioinformatics, biophysics, biotechnology, genetics, geobotany, human biology, marine biology, microbiology, molecular biology, cellular biology, zoology, bioinorganic chemistry, microchemistry and environmental chemistry.
Newest articles

Webb captures top of iconic horsehead nebula in unprecedented detail
NASA’s James Webb Space Telescope has captured the sharpest infrared images to date of a zoomed-in portion of one of the most distinctive objects in our skies, the Horsehead Nebula….
Cost-effective, high-capacity, and cyclable lithium-ion battery cathodes
Charge-recharge cycling of lithium-superrich iron oxide, a cost-effective and high-capacity cathode for new-generation lithium-ion batteries, can be greatly improved by doping with readily available mineral elements. The energy capacity and…
Novel genetic plant regeneration approach
…without the application of phytohormones. Researchers develop a novel plant regeneration approach by modulating the expression of genes that control plant cell differentiation. For ages now, plants have been the…





















