A Larger Pocket
A single targeted mutation is enough to alter a natural peptide system so that it also incorporates non-natural amino acids into peptides, report Swiss scientists in the journal Angewandte Chemie.
The mutation increases the size of the binding cavity in one domain of the system, which changes the substrate specificity. The researchers are thus able to incorporate amino acids with a specific reactive group that can later be used to easily modify the peptide.
In the search for new pharmaceuticals through the use of combinatorial chemistry and screening processes, researchers are often faced with the task of modifying and varying natural substances—sometimes by adding further molecular components, for example.
Highly specific coupling with molecular markers is particularly important because it allows scientists to monitor the distribution of natural substances in cells and tissues. Coupling reactions that are almost as snapping components together can be carried out by a technique known as “click chemistry”. This method encompasses broadly applicable reactions like those between alkynes and azides, which deliver high yields.
For this technique, the natural substance must first be equipped with such an alkyne or azide group. One way to achieve this would be through the incorporation of amino acids with alkyne or azide side chains into proteins through alteration of their biosynthesis.
However, many interesting natural substances, such as the gramicidin antibiotics, are not formed by way of the classical pathways of protein biosynthesis through the reading of genes and the assembly of amino acids in the ribosomes. Instead, they are made by nonribosomal peptide synthetases, very large multi-enzyme complexes whose individual modules hang together like pearls on a necklace.
These activate the amino acid building blocks and incorporate them into the growing peptide chain. The number, type, and ordering of the individual modules determine the length and composition of the resulting—usually short-chained—peptide. In addition to the usual amino acids, it is also possible to incorporate other, sometimes unusual, individual building blocks, which allows for the formation of an astonishingly large variety of peptides.
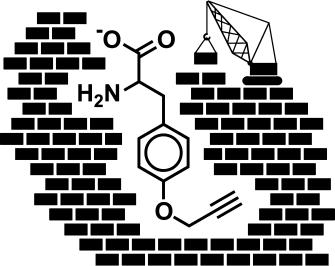
Researchers working with Donald Hilvert at the ETH in Zurich exchanged an individual amino acid in one module of the nonribosomal production apparatus for the antibiotic gramicidin S through a mutation. This altered an area known as an A domain, which specifically recognizes and activates the natural amino acid phenylalanine.
The mutation causes the binding cavity to be roomier, so that non-natural amino acids that contain an azide or alkyne group can be activated and incorporated into the peptide chain in place of phenylalanine. The catalytic activity of the overall system is not affected by this change in selectivity.
Because many different nonribosomal synthesis systems contain such A domains, this new method is potentially a general approach for equipping important natural substances with a reactive site for highly specific covalent modification.
About the Author
Dr. Donald Hilvert is Professor of Chemistry at the ETH Zürich. His research group is investigating how enzymes work and evolve and applying this knowledge to the design of new protein-based catalysts. These efforts have been recognized by a number of awards, including the Pfizer Award in Enzyme Chemistry and the Emil Thomas Kaiser Award from the Protein Society.
Author: Donald Hilvert, ETH Zürich (Switzerland), http://www.protein.ethz.ch
Title: Reprogramming Nonribosomal Peptide Synthetases for “Clickable” Amino Acids
Angewandte Chemie International Edition, Permalink to the article: http://dx.doi.org/10.1002/anie.201405281
Media Contact
More Information:
http://pressroom.angewandte.orgAll latest news from the category: Life Sciences and Chemistry
Articles and reports from the Life Sciences and chemistry area deal with applied and basic research into modern biology, chemistry and human medicine.
Valuable information can be found on a range of life sciences fields including bacteriology, biochemistry, bionics, bioinformatics, biophysics, biotechnology, genetics, geobotany, human biology, marine biology, microbiology, molecular biology, cellular biology, zoology, bioinorganic chemistry, microchemistry and environmental chemistry.
Newest articles

Trotting robots reveal emergence of animal gait transitions
A four-legged robot trained with machine learning by EPFL researchers has learned to avoid falls by spontaneously switching between walking, trotting, and pronking – a milestone for roboticists as well…

Innovation promises to prevent power pole-top fires
Engineers in Australia have found a new way to make power-pole insulators resistant to fire and electrical sparking, promising to prevent dangerous pole-top fires and reduce blackouts. Pole-top fires pose…

Possible alternative to antibiotics produced by bacteria
Antibacterial substance from staphylococci discovered with new mechanism of action against natural competitors. Many bacteria produce substances to gain an advantage over competitors in their highly competitive natural environment. Researchers…