Nanoscale factories built to order
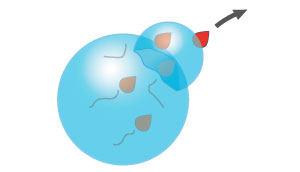
Understanding how radical fragments (red spheres) react with surrounding water molecules to create hydroxyl radicals, while leaving behind hydrophobic residues (black), can help green chemistry researchers. © 2016 A*STAR Institute of Chemical and Engineering Sciences
A discovery led by Singapore's Agency for Science, Technology and Research (A*STAR) could lead to improvements in the way drugs are delivered to the right parts of the body by uncovering the mechanisms that help oil, water, and free radicals mix in tiny droplets [1][2].
Emulsion polymerization is an emerging technology used to produce enormous chain-like molecules called polymers inside oil-filled drops suspended in water. This approach enables producers of goods such as latex paints to do away with traditional oil-based solvents, which helps them meet stricter environmental controls. Recently, researchers have discovered that ‘mini-emulsions’, in which droplets are shrunk to nanoscale sizes using powerful blenders and stabilized with fatty molecules, can produce nanoparticles for applications including controlled drug release.
Alex van Herk from the A*STAR Institute of Chemical and Engineering Sciences explains that in mini-emulsions, each droplet can be regarded as a ‘nanoreactor’ — a segregated system where all the ingredients for polymerization are present in one spot. Once a highly reactive free radical enters the drop, the small molecules inside link into chains. “The nanoreactors grow completely independently, and we can achieve very high reaction rates,” he says.
This polymerization only works when one free radical enters a nanoreactor. However, the molecules that generate free radicals, known as initiators, generally produce them in pairs. To better understand these radical movements, van Herk and colleagues from the Netherlands and the United Kingdom investigated the effects of using initiators that either repelled or attracted water molecules.
Typical initiators are water-soluble and researchers propose that they create pairs of free radicals in water where one of the free radicals enters the nanoreactor and starts the polymerization. However, when the initiator is a water-repelling molecule, such as lauroyl peroxide, theory predicts the chemical reaction will be hindered because the two radicals in a confined space would easily recombine and the polymerization process would not start.
Surprisingly, the A*STAR-led team found mini-emulsion polymerization proceeded rapidly and completely using lauroyl peroxide initiators. To explain this discrepancy, the team deduced that a free radical must leave by an alternative mechanism, known as chain transfer, which transforms one of the water molecules surrounding the nanoreactor into a hydroxyl radical compound. The remaining radical produces latex nanoparticles that correspond one-to-one with the initial droplet size — a benefit for manufacturers seeking to predict morphologies with exact specifications.
“Industry is only modestly adopting mini-emulsion polymerization, partly because its mechanism is not fully understood and controllable yet,” says van Herk. “These findings give us a better edge to design and produce special nanoparticle morphologies such as low-cost nanocapsules.”
The A*STAR-affiliated researchers contributing to this research are from the Institute of Chemical and Engineering Sciences. For more information about the team’s research, please visit the Polymer Engineering & Characterisation group webpage.
Associated links
Journal information
[1] Jansen, T. G. T., Meuldijk, J., Lovell, P. A. & van Herk, A. M. On the miniemulsion polymerization of very hydrophobic monomers initiated by a completely water-insoluble initiator: thermodynamics, kinetics, and mechanism. Journal of Polymer Science Part A: Polymer Chemistry 54, 2731–2745 (2016).
[2] Jansen, T. G. T., Meuldijk, J., Lovell, P. A. & van Herk, A. M. On the reaction characteristics of miniemulsion polymerization with aqueous phase initiation — Experiments and modeling. Macromolecular Reaction Engineering 9, 19–31 (2015).
Media Contact
All latest news from the category: Life Sciences and Chemistry
Articles and reports from the Life Sciences and chemistry area deal with applied and basic research into modern biology, chemistry and human medicine.
Valuable information can be found on a range of life sciences fields including bacteriology, biochemistry, bionics, bioinformatics, biophysics, biotechnology, genetics, geobotany, human biology, marine biology, microbiology, molecular biology, cellular biology, zoology, bioinorganic chemistry, microchemistry and environmental chemistry.
Newest articles

Microscopic basis of a new form of quantum magnetism
Not all magnets are the same. When we think of magnetism, we often think of magnets that stick to a refrigerator’s door. For these types of magnets, the electronic interactions…

An epigenome editing toolkit to dissect the mechanisms of gene regulation
A study from the Hackett group at EMBL Rome led to the development of a powerful epigenetic editing technology, which unlocks the ability to precisely program chromatin modifications. Understanding how…

NASA selects UF mission to better track the Earth’s water and ice
NASA has selected a team of University of Florida aerospace engineers to pursue a groundbreaking $12 million mission aimed at improving the way we track changes in Earth’s structures, such…





















