Plastics, fuels and chemical feedstocks from CO2? They're working on it
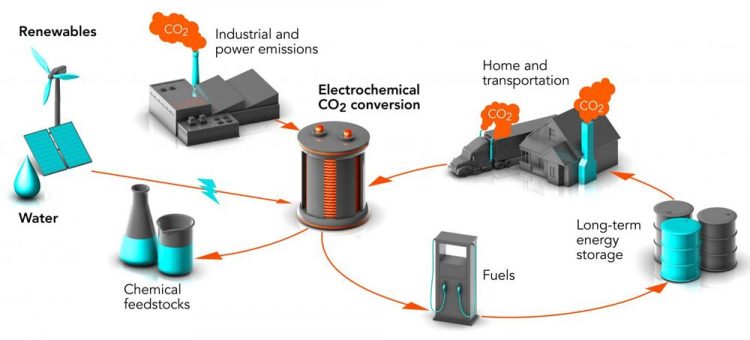
Researchers at Stanford and SLAC are working on ways to convert waste carbon dioxide (CO2) into chemical feedstocks and fuels, turning a potent greenhouse gas into valuable products. The process is called electrochemical conversion. When powered by renewable energy sources, it could reduce levels of carbon dioxide in the air and store energy from these intermittent sources in a form that can be used any time. Credit: Greg Stewart/SLAC National Accelerator Laboratory
One way to reduce the level of carbon dioxide in the atmosphere, which is now at its highest point in 800,000 years, would be to capture the potent greenhouse gas from the smokestacks of factories and power plants and use renewable energy to turn it into things we need, says Thomas Jaramillo.
As director of SUNCAT Center for Interface Science and Catalysis, a joint institute of Stanford University and the Department of Energy's SLAC National Accelerator Laboratory, he's in a position to help make that happen.
A major focus of SUNCAT research is finding ways to transform CO2 into chemicals, fuels, and other products, from methanol to plastics, detergents and synthetic natural gas. The production of these chemicals and materials from fossil fuel ingredients now accounts for 10% of global carbon emissions; the production of gasoline, diesel, and jet fuel accounts for much, much more.
“We have already emitted too much CO2, and we're on track to continue emitting it for years, since 80% of the energy consumed worldwide today comes from fossil fuels,” says Stephanie Nitopi, whose SUNCAT research is the basis of her newly acquired Stanford PhD.
“You could capture CO2 from smokestacks and store it underground,” she says. “That's one technology currently in play. An alternative is to use it as a feedstock to make fuels, plastics, and specialty chemicals, which shifts the financial paradigm. Waste CO2 emissions now become something you can recycle into valuable products, providing a new incentive to reduce the amount of CO2 released into the atmosphere. That's a win-win.”
We asked Nitopi, Jaramillo, SUNCAT staff scientist Christopher Hahn and postdoctoral researcher Lei Wang to tell us what they're working on and why it matters.
First the basics: How do you convert CO2 into these other products?
Tom: It's essentially a form of artificial photosynthesis, which is why DOE's Joint Center for Artificial Photosynthesis funds our work. Plants use solar energy to convert CO2 from the air into carbon in their tissues. Similarly, we want to develop technologies that use renewable energy, like solar or wind, to convert CO2 from industrial emissions into carbon-based products.
Chris: One way to do this is called electrochemical CO2 reduction, where you bubble CO2 gas up through water and it reacts with the water on the surface of a copper-based electrode. The copper acts as a catalyst, bringing the chemical ingredients together in a way that encourages them to react. Put very simply, the initial reaction strips an oxygen atom from CO2 to form carbon monoxide, or CO, which is an important industrial chemical in its own right. Then other electrochemical reactions turn CO into important molecules such as alcohols, fuels and other things.
Today this process requires a copper-based catalyst. It's the only one known to do the job. But these reactions can produce numerous products, and separating out the one you want is costly, so we need to identify new catalysts that are able to guide the reaction toward making only the desired product.
How so?
Lei: When it comes to improving a catalyst's performance, one of the key things we look at is how to make them more selective, so they generate just one product and nothing else. About 90 percent of fuel and chemical manufacturing depends on catalysts, and getting rid of unwanted byproducts is a big part of the cost.
We also look at how to make catalysts more efficient by increasing their surface area, so there are a lot more places in a given volume of material where reactions can occur simultaneously. This increases the production rate.
Recently we discovered something surprising: When we increased the surface area of a copper-based catalyst by forming it into a flaky “nanoflower” shape, it made the reaction both more efficient and more selective. In fact, it produced virtually no byproduct hydrogen gas that we could measure. So this could offer a way to tune reactions to make them more selective and cost-competitive.
Stephanie: This was so surprising that we decided to revisit all the research we could find on catalyzing electrochemical CO2 conversion with copper, and the many ways people have tried to understand and fine-tune the process, using both theory and experiments, going back four decades. There's been an explosion of research on this – about 60 papers had been published as of 2006, versus more than 430 out there today – and analyzing all the studies with our collaborators at the Technical University of Denmark took two years.
We were trying to figure out what makes copper special, why it's the only catalyst that can make some of these interesting products, and how we can make it even more efficient and selective – what techniques have actually pushed the needle forward? We also offered our perspectives on promising research directions.
One of our conclusions confirms the results of the earlier study: The copper catalyst's surface area can be used to improve both the selectivity and overall efficiency of reactions. So this is well worth considering as a chemical production strategy.
Does this approach have other benefits?
Tom: Absolutely. If we use clean, renewable energy, like wind or solar, to power the controlled conversion of waste CO2 to a wide range of other products, this could actually draw down levels of CO2 in the atmosphere, which we will need to do to stave off the worst effects of global climate change.
Chris: And when we use renewable energy to convert CO2 to fuels, we're storing the variable energy from those renewables in a form that can be used any time. In addition, with the right catalyst, these reactions could take place at close to room temperature, instead of the high temperatures and pressures often needed today, making them much more energy efficient.
How close are we to making it happen?
Tom: Chris and I explored this question in a recent Perspective article in Science, written with researchers from the University of Toronto and TOTAL American Services, which is an oil and gas exploration and production services firm.
We concluded that renewable energy prices would have to fall below 4 cents per kilowatt hour, and systems would need to convert incoming electricity to chemical products with at least 60% efficiency, to make the approach economically competitive with today's methods.
Chris: This switch couldn't happen all at once; the chemical industry is too big and complex for that. So one approach would be to start with making high-value, high-volume products like ethylene, which is used to make alcohols, polyester, antifreeze, plastics and synthetic rubber. It's a $230 billion global market today. Switching from fossil fuels to CO2 as a starting ingredient for ethylene in a process powered by renewables could potentially save the equivalent of about 860 million metric tons of CO2 emissions per year.
The same step-by-step approach applies to sources of CO2. Industry could initially use relatively pure CO2 emissions from cement plants, breweries or distilleries, for instance, and this would have the side benefit of decentralizing manufacturing. Every country could provide for itself, develop the technology it needs, and give its people a better quality of life.
Tom: Once you enter certain markets and start scaling up the technology, you can attack other products that are tougher to make competitively today. What this paper concludes is that these new processes have a chance to change the world.
###
Citations:
L. Wang et al., Nature Catalysis, 17 June 2019 (10.1038/s41929-019-0301-z)
S. Nitopi et al., Chemical Reviews, 22 May 2019 (10.1021/acs.chemrev.8b00705)
P. De Luna et al., Science, 26 April 2019 (10.1126/science.aav3506)
Media Contact
All latest news from the category: Ecology, The Environment and Conservation
This complex theme deals primarily with interactions between organisms and the environmental factors that impact them, but to a greater extent between individual inanimate environmental factors.
innovations-report offers informative reports and articles on topics such as climate protection, landscape conservation, ecological systems, wildlife and nature parks and ecosystem efficiency and balance.
Newest articles

A universal framework for spatial biology
SpatialData is a freely accessible tool to unify and integrate data from different omics technologies accounting for spatial information, which can provide holistic insights into health and disease. Biological processes…

How complex biological processes arise
A $20 million grant from the U.S. National Science Foundation (NSF) will support the establishment and operation of the National Synthesis Center for Emergence in the Molecular and Cellular Sciences (NCEMS) at…

Airborne single-photon lidar system achieves high-resolution 3D imaging
Compact, low-power system opens doors for photon-efficient drone and satellite-based environmental monitoring and mapping. Researchers have developed a compact and lightweight single-photon airborne lidar system that can acquire high-resolution 3D…





















