Martian salts must touch ice to make liquid water, study shows
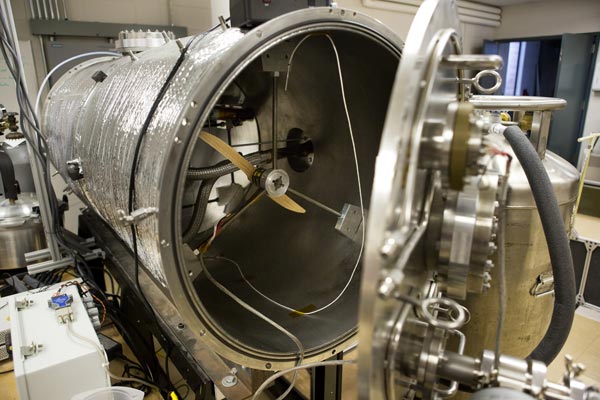
A Mars Atmospheric Chamber in the Space Research Building at the University of Michigan on June 18, 2014. The chamber simulates the atmospheric conditions of Mars in hopes of producing water through the interaction of salt with the atmospheric conditions simulated by the chamber. The resulting research allows Astrobiologists to postulate about the potential of life on Mars. Credit: Joseph Xu, Michigan Engineering Communications & Marketing
Liquid water is an essential ingredient for life as we know it. Mars is one of the very few places in the solar system where scientists have seen promising signs of it – in gullies down crater rims, in instrument readings, and in Phoenix spacecraft self portraits that appeared to show wet beads on the lander’s leg several years ago.
No one has directly detected liquid water beyond Earth, though. Experiments at the University of Michigan are among the first to test theories about how it could exist in a climate as cold as Mars’.
The researchers found that a type of salt present in Martian soil can readily melt ice it touches – just like salts do on Earth’s slippery winter walkways and roads. But this Martian salt cannot, as some scientists suggested, form liquid water by sucking vapor out of the air through a process called deliquescence.
“For me, the most exciting thing is that I can now understand how the droplets formed on the Phoenix leg,” said Nilton Renno, a professor of atmospheric, oceanic and space sciences at the University of Michigan in Ann Arbor who led the research. The new research is detailed in a paper accepted for publication in Geophysical Research Letters, a journal of the American Geophysical Union.
In 2008, Renno was the first to notice strange globules in photos Phoenix sent back. Over several weeks, the globules seemed to grow and coalesce. While Renno deemed them water and suggested that salts on the planet’s surface might make it so, many of his colleagues disagreed. Salts had never been found on Mars.
But then they were. Among those that Phoenix detected is calcium perchlorate, a mixture of calcium, chlorine and oxygen that’s found in arid places like the Atacama Desert in Chile. Years later, the Curiosity rover found it elsewhere on Mars. Now scientists believe it and other salts are sprinkled across the planet’s surface.
In the case of Phoenix, Renno believes the craft’s landing thrusters blasted away the topsoil, exposing the ice and melting it. That formed muddy saltwater that splashed on the lander’s leg as it touched down in the northern polar region. The salts allowed the droplets to remain liquid. Their existence and stability, Renno says, tipped scientists off to a cycle that doesn’t need always need help from an Earth-borne spacecraft.
The U-M researchers recreated the Phoenix landing site conditions in their lab in metal cylinders two feet high and five feet long. The late Martian spring/early summer temperatures in the chambers ranged from -121 degrees to -21 degrees Celsius (-185 to -5 Fahrenheit). Atmospheric pressure hovered around 1 percent of Earth’s. Relative humidity varied, but for most experiments, it was set to 100 percent.
They tested two scenarios: perchlorate by itself and perchlorate on top of water ice. In the perchlorate-only experiments, they put millimeter-thick layers of salt on a temperature-controlled plate or Mars-like soil. Even after more than three hours, no liquid water formed. That told them deliquescence wasn’t occurring, and isn’t likely to be a significant process on Mars.
When the researchers placed calcium perchlorate or salty soil directly on a 3-millimeter-thick ice layer, drops of liquid water formed within minutes when the chambers reached -73 degrees C (-100 F). That’s well within the range of conditions observed at the Phoenix landing site.
Researchers didn’t rely on their eyes or cameras to be sure liquid water was there. They used a technique called Raman scattering spectroscopy that involves shining lasers onto the surface and examining the reflected light. Different substances and states of matter have different reflective signatures.
The findings show how small amounts of liquid water could exist across a large swath of Mars’ surface and shallow subsurface, from its polar regions to its mid-latitudes, for several hours a day during the spring and early summer. Such a cycle could form gullies, Renno says, flowing, freezing, thawing and flowing again. Water could also form just beneath the surface.
Renno says the water wouldn’t necessarily need to stay liquid indefinitely for it to support microbial life now or have supported it in the past. Antarctic saltwater and lattices of brine-filled ice-combs have been found to harbor microbial organisms on Earth.
“Mars is the planet in our solar system that is most similar to Earth. Studies suggest that Mars used to be even more Earth-like in the past, with flowing water on the surface. By studying the formation of liquid water on Mars we can learn about possibilities of life outside Earth and look for resources for future missions,” said Erik Fischer, doctoral student in the Department of Atmospheric, Oceanic and Space Sciences (AOSS) at the University of Michigan and first author of the new paper.
The research is supported by NASA’s Exobiology Program. In addition to Renno and Fischer, other authors are Harvey Elliott, doctoral student in AOSS and Germán M. Martínez, research scientist at AOSS.
For additional images, visit https://www.flickr.com/photos/michigan-engineering/sets/72157645284722303/
For more information, visit “Revisiting Mars: The search for liquid water and life on the planet next door”: http://www.engin.umich.edu/college/about/news/stories/2011/june/revisiting-mars-the-search-for-liquid-water-and-life-on-the-planet-next-door
Notes for Journalists
Journalists and public information officers (PIOs) of educational and scientific institutions who have registered with AGU can download a PDF copy of this accepted article by clicking on this link: http://onlinelibrary.wiley.com/doi/10.1002/2014GL060302/abstract
Or, you may order a copy of the final paper by emailing your request to Nanci Bompey at nbompey@agu.org. Please provide your name, the name of your publication, and your phone number.
Neither the paper nor this press release is under embargo.
Title
“Experimental evidence for the formation of liquid saline water on Mars”
Authors:
Erik Fischer, Germán M. Martínez, Harvey M. Elliott and Nilton O. Rennó: Department of Atmospheric, Oceanic and Space Sciences, University of Michigan, Ann Arbor, Michigan, USA.
Contact information for the authors:
Erik Fischer: +1 (734) 647-9441, erikfis@umich.edu
Nanci Bompey
+1 (202) 777-7524
nbompey@agu.org
University of Michigan Contact:
Nicole Casal Moore
+1 (734) 647-7087
ncmoore@umich.edu
Media Contact
All latest news from the category: Earth Sciences
Earth Sciences (also referred to as Geosciences), which deals with basic issues surrounding our planet, plays a vital role in the area of energy and raw materials supply.
Earth Sciences comprises subjects such as geology, geography, geological informatics, paleontology, mineralogy, petrography, crystallography, geophysics, geodesy, glaciology, cartography, photogrammetry, meteorology and seismology, early-warning systems, earthquake research and polar research.
Newest articles

A universal framework for spatial biology
SpatialData is a freely accessible tool to unify and integrate data from different omics technologies accounting for spatial information, which can provide holistic insights into health and disease. Biological processes…

How complex biological processes arise
A $20 million grant from the U.S. National Science Foundation (NSF) will support the establishment and operation of the National Synthesis Center for Emergence in the Molecular and Cellular Sciences (NCEMS) at…

Airborne single-photon lidar system achieves high-resolution 3D imaging
Compact, low-power system opens doors for photon-efficient drone and satellite-based environmental monitoring and mapping. Researchers have developed a compact and lightweight single-photon airborne lidar system that can acquire high-resolution 3D…





















