Solid or liquid – the particle size matters
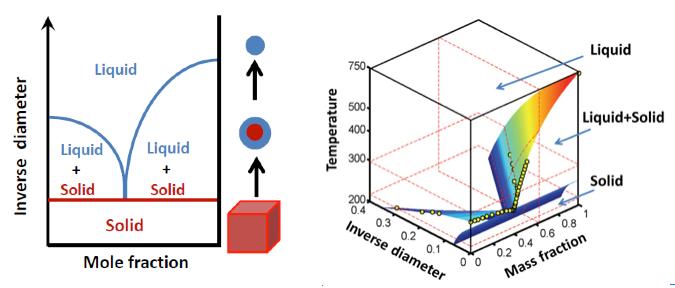
The particle size of aerosol nanoparticles is as important for the phase state as the chemical composition and temperture. The 2D (left) and 3D (right) phase diagram illustrates this correlation. Hang Su, MPI for Chemistry
Whether tiny particles in the air, so-called aerosol nanoparticles, are solid or liquid, is of great importance to atmospheric and climate scientists. The phase state determines if and how fast such particles grow into cloud condensation nuclei on which water vapor can condense to form cloud droplets and precipitation.
Until recently, however, experimental observations of the solid-liquid phase transitions and humidity-dependent growth of atmospheric aerosol nanoparticles could not be explained by theoretical calculations and model predictions.
Scientists at the Max Planck Institute for Chemistry could now resolve the riddle. “The particle size is more important than we previously thought, “says Yafang Cheng, group leader at the institute in Mainz. “For example, salt particles can become liquid not only by increasing temperature or humidity, but also by reducing the particle size,” says the lead author of a recent publication in Nature Communications.
The researchers around Yafang Cheng and Hang Su analyzed high precision measurement data on the hygroscopic growth of sodium chloride and ammonium sulfate nanoparticles exposed to varying relative humidity.
From these growth curves, the researchers calculated the interfacial energies and critical diameters for the solid-liquid phase transitions of the salt nanoparticles. According to similar analyses, the researchers expect that organic aerosol particles commonly occuring in large quantities in the atmosphere are always liquid at room temperature when their diameter is below approximately 20 nanometers.
“Based on our results the particle size should be considered as an additional dimension in the phase diagram of aerosol nanoparticles,” says Cheng´s colleague Hang Su. “Our findings are also relevant for other research areas where nanoparticles play a role, including the biomedical and materials sciences.” For example, they may help to determine and control the solubility and concentration of therapeutic or reactive agents in in synthetic nanoparticles for medical or technical applications.
Original publication
Cheng et al., Size dependence of phase transitions in aerosol nanoparticles, Nature Comm., 6, 2015, doi:10.1038/ncomms6923
http://www.nature.com/ncomms/2015/150114/ncomms6923/full/ncomms6923.html
Contact
Dr. Hang Su
Max Planck Institute for Chemistry
Telephone: +49-6131-3057301
E-Mail: h.su@mpic.de
Weitere Informationen:
http://www.mpic.de/en/news/press-information/news/solid-or-liquid-the-particle-s…
Media Contact
All latest news from the category: Life Sciences and Chemistry
Articles and reports from the Life Sciences and chemistry area deal with applied and basic research into modern biology, chemistry and human medicine.
Valuable information can be found on a range of life sciences fields including bacteriology, biochemistry, bionics, bioinformatics, biophysics, biotechnology, genetics, geobotany, human biology, marine biology, microbiology, molecular biology, cellular biology, zoology, bioinorganic chemistry, microchemistry and environmental chemistry.
Newest articles

Sea slugs inspire highly stretchable biomedical sensor
USC Viterbi School of Engineering researcher Hangbo Zhao presents findings on highly stretchable and customizable microneedles for application in fields including neuroscience, tissue engineering, and wearable bioelectronics. The revolution in…

Twisting and binding matter waves with photons in a cavity
Precisely measuring the energy states of individual atoms has been a historical challenge for physicists due to atomic recoil. When an atom interacts with a photon, the atom “recoils” in…

Nanotubes, nanoparticles, and antibodies detect tiny amounts of fentanyl
New sensor is six orders of magnitude more sensitive than the next best thing. A research team at Pitt led by Alexander Star, a chemistry professor in the Kenneth P. Dietrich…





















