Chemists connect three components with new coupling reaction
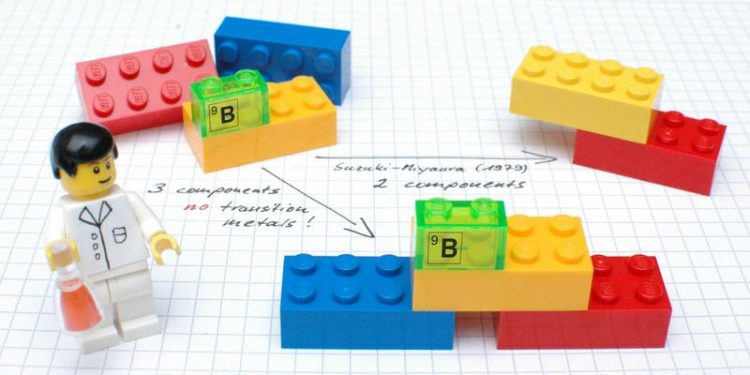
The new reaction, explained using plastic building bricks: In a single reaction, three (bottom) instead of two (top right) chemical components are linked via carbon-carbon bonds. Photo: WWU/Ludger Tebben
In the current issue of the “Science” magazine, chemists at Münster University present a new approach which for the first time enables three – and not, as previously, two – chemical components to be “coupled” in one single reaction, without any metals to aid the process.
The researchers succeeded in producing not only pharmaceutically relevant compounds containing fluorine, but also various γ-lactones. These organic compounds occur widely in various types of fruit and also, for example, as flavouring substances in whisky and cognac.
“What is remarkable is that for the reaction process no expensive transition metals are needed as catalysts,” says Prof. Armido Studer from the Institute of Organic Chemistry at Münster University, the lead author of the study.
This represents an important further development of the classic variant, he says, especially with a view to the increasing relevance of sustainable, environmentally-friendly chemistry – so-called green chemistry.
The background: one of the greatest challenges for organic chemists is to create specific bonds between carbon atoms in various chemical components. This is, however, essential for the construction of complex, pharmaceutically active and biologically relevant molecules. “The tools which are particularly important for this are so-called cross-coupling reactions,” Studer explains.
Probably the most famous example, he adds, is the “Suzuki-Miyaura coupling”, which was awarded the Nobel Prize for Chemistry in 2010. This reaction, used by the chemical industry in tonne scale, makes it possible to link two chemical components, although one of the components has to contain a reactive boron moiety.
What is decisive for the reaction process, says Studer, is the presence of expensive transition metals such as palladium, which brings the two reactants together, so that in the end a carbon-carbon bond is formed.
The method now developed by the Münster chemists includes the formation of two carbon-carbon bonds. “Unlike classic cross-couplings, however, the valuable boron moiety remains in the product,” Studer explains. “At this point, further changes can then be made to the molecules in the same reaction vessel.” This method makes it possible, he adds, to produce a large number of different derivatives.
The researchers received financial support from the European Research Council (ERC) for their work.
Original publication:
Marvin Kischkewitz, Kazuhiro Okamoto, Christian Mück-Lichtenfeld, Armido Studer (2017): Radical-polar crossover reactions of vinylboron ate complexes. Science Vol. 355, Issue 6328, pp 936-938; DOI: 10.1126/science.aal3803
https://www.uni-muenster.de/Chemie.oc/studer/en/members.html Studer Research Group at Münster University
http://science.sciencemag.org/content/355/6328/936 Original publication
Media Contact
All latest news from the category: Life Sciences and Chemistry
Articles and reports from the Life Sciences and chemistry area deal with applied and basic research into modern biology, chemistry and human medicine.
Valuable information can be found on a range of life sciences fields including bacteriology, biochemistry, bionics, bioinformatics, biophysics, biotechnology, genetics, geobotany, human biology, marine biology, microbiology, molecular biology, cellular biology, zoology, bioinorganic chemistry, microchemistry and environmental chemistry.
Newest articles

Sea slugs inspire highly stretchable biomedical sensor
USC Viterbi School of Engineering researcher Hangbo Zhao presents findings on highly stretchable and customizable microneedles for application in fields including neuroscience, tissue engineering, and wearable bioelectronics. The revolution in…

Twisting and binding matter waves with photons in a cavity
Precisely measuring the energy states of individual atoms has been a historical challenge for physicists due to atomic recoil. When an atom interacts with a photon, the atom “recoils” in…

Nanotubes, nanoparticles, and antibodies detect tiny amounts of fentanyl
New sensor is six orders of magnitude more sensitive than the next best thing. A research team at Pitt led by Alexander Star, a chemistry professor in the Kenneth P. Dietrich…





















