Broadening the scope for synthesizing optically active compounds
Chiral compounds are increasingly important in chemical manufacturing. They are distinguished by a special kind of asymmetry in their molecular structure.
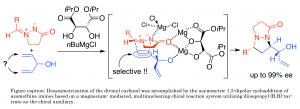
Yutaka Ukaji and colleagues at Kanazawa University have now developed a method for desymmetrising compounds to produce new chiral molecules. The process allows 99% selectivity in the chemicals produced. http://www.kanazawa-u.ac.jp/research_bulletin/index.html
The property of chirality is defined by the existence of distinct mirror image geometric arrangements of the constituent parts of a molecule, known as stereoisomers. Just as your right hand cannot be directly superimposed on the left, if the molecule is chiral the mirror images cannot be directly superimposed. Chiral compounds are often described as optically active as one stereoisomer will rotate the plane of incident polarised light to the left and the other will rotate it to the right.
Desymmetrisation methods to produce chiral compounds exist but the range of compounds amenable to the approach remains limited. Ukaji and his colleagues focused on a type of organic compound known as divinyl carbinols – where the vinyl group describes an ethylene molecular group and the carbinol describes an alcohol derived from methanol. Desymmetrisation of divinyl carbinols can provide new optically active alcohol derivatives that contain useful functional groups for further chemical transformations.
The approach developed by the Kanazawa team built on previous work demonstrating an asymmetric ‘cycloaddition’ reaction where compounds with unsaturated (double, triple etc) bonds combine forming a ring. Their current work demonstrates the reaction on divinyl carbinols with selective production of one mirror image product over the other of over 99%.
They conclude in their report on the work, “This method would be useful for the preparation of optically active nitrogen- and oxygen containing chemicals.”
Further information:
Organization of Frontier Science and Innovation
Kanazawa University
Kakuma, Kanazawa, Ishikawa 920-1192, Japan
E-mail: fsojimu@adm.kanazawa-u.ac.jp
Website: http://www.o-fsi.kanazawa-u.ac.jp/en
About Kanazawa University
Kanazawa University, Japan publishes the May 2014 issue of its online newsletter, Kanazawa University Research Bulletin: http://www.kanazawa-u.ac.jp/research_bulletin/index.html
Kanazawa University Research Bulletin highlights the latest research from one of Japan's leading comprehensive universities with its three colleges and 16 schools offering courses in subjects that include medicine, computer engineering, and humanities.
As the leading comprehensive university on the Sea of Japan coast, Kanazawa University has contributed greatly to higher education and academic research in Japan since it was founded in 1949. The University has three colleges and 16 schools offering courses in subjects that include medicine, computer engineering, and humanities.
The University is located on the coast of the Sea of Japan in Kanazawa—a city rich in history and culture. The city of Kanazawa has cultivated a highly respected intellectual profile since the time of the Kaga fiefdom (1598–1867). Kanazawa University is divided into two main campuses: Kakuma and Takaramachi for its approximately 12,200 students including 500 from overseas.
Kanazawa University website: http://www.kanazawa-u.ac.jp/e/index.html
Associated links
Journal information
Mari Yoshida, Naotaro Sassa, Tomomitsu Kato, Shuhei Fujinami, Takahiro Soeta,
Katsuhiko Inomata, and Yutaka Ukaji*
Desymmetrization of 1,4-pentadien-3-ol by the asymmetric 1,3-
dipolar cycloaddition of azomethine imines. Chem. Eur. J. 20 (2014) 2058–2064.
DOI: 10.1002/chem.201302889
Division of Material Sciences, Graduate School of Natural Science and Technology,
Kanazawa University, Kakuma, Kanazawa, Ishikawa 920-1192, Japan
*corresponding author, e-mail address: ukaji@staff.kanazawa-u.ac.jp
Media Contact
All latest news from the category: Process Engineering
This special field revolves around processes for modifying material properties (milling, cooling), composition (filtration, distillation) and type (oxidation, hydration).
Valuable information is available on a broad range of technologies including material separation, laser processes, measuring techniques and robot engineering in addition to testing methods and coating and materials analysis processes.
Newest articles

A universal framework for spatial biology
SpatialData is a freely accessible tool to unify and integrate data from different omics technologies accounting for spatial information, which can provide holistic insights into health and disease. Biological processes…

How complex biological processes arise
A $20 million grant from the U.S. National Science Foundation (NSF) will support the establishment and operation of the National Synthesis Center for Emergence in the Molecular and Cellular Sciences (NCEMS) at…

Airborne single-photon lidar system achieves high-resolution 3D imaging
Compact, low-power system opens doors for photon-efficient drone and satellite-based environmental monitoring and mapping. Researchers have developed a compact and lightweight single-photon airborne lidar system that can acquire high-resolution 3D…





















