When gold becomes a catalyst
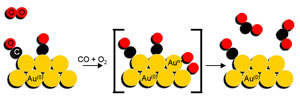
Mechanism for the catalytic reaction 2CO + O2 -> 2CO2
The team used nano-particles of gold instead of bulk gold. The catalyst structure looks as if someone had pulverized a piece of gold and spread the tiny nano-sized pieces over an aluminum oxide support. The properties of the nano-particles are very different from those of bulk gold. Only when the gold atoms are confined to the size of just a few millionth of a millimetre they start showing the desired catalytic behaviour.
Scientists already knew that gold nano-particles react with this kind of setup and catalyses CO with oxygen (O2) into CO2. What they did not know was how the oxygen is activated on the catalyst. In order to find that out, they set up a cell where they could carry out the reaction, and in situ perform an X-ray experiment with the ESRF beam.
The researchers first applied a flow of oxygen over the gold nano-particles and observed how the oxygen becomes chemically active when bound on the gold nano-particles using high-energy resolution X-ray absorption spectroscopy. While constantly monitoring the samples, they switched to a flow of toxic carbon monoxide and found that the oxygen bound to the gold reacted with the carbon monoxide to form carbon dioxide. Without the gold nano-particles, this reaction does not take place. “We knew beforehand that the small gold particles were active, but not how they did the reaction. The nice thing is that we have been able to observe, for the first time, the steps and path of the reaction. The results followed almost perfectly our original hypotheses. Isn’t it beautiful that the most inert bulk metal is so reactive when finely dispersed?” comments Jeroen A. van Bokhoven, the corresponding author of the paper.
The possible applications of this research could involve pollution control such as air cleaning, or purification of hydrogen streams used for fuel cells. “Regarding the technique we used, the exceptionally high structural detail that can be obtained with it could be used to study other catalytic systems, with the aim of making them more stable and perform better”, says van Bokhoven.
One of the great advantages of this experiment is the nature of catalysis. The fact that once the material has reacted, it goes back to its initial state, has made the experiments easier. Nevertheless, in technological terms, it has been very demanding: “We combined the unique properties of our beamline with an interesting and strongly debated question in catalysis. Some extra time was needed to adapt the beamline, to the special requirements of this experiment,” explains Pieter Glatzel, scientist in charge of ID26 beamline, where the experiments were carried out. At the end, it only took the team a bit over half a year to prepare and carry out the experiments and publish the paper. “This is a very nice recognition of our work,” says Glatzel.
The article appears in this week’s international edition of Angewandte Chemie with a very high impact among the chemistry audience. In addition to this, the paper has been attributed the status of Very Important Paper, which is given to only 5% of all the publications in this journal.
Media Contact
More Information:
http://www.esrf.frAll latest news from the category: Physics and Astronomy
This area deals with the fundamental laws and building blocks of nature and how they interact, the properties and the behavior of matter, and research into space and time and their structures.
innovations-report provides in-depth reports and articles on subjects such as astrophysics, laser technologies, nuclear, quantum, particle and solid-state physics, nanotechnologies, planetary research and findings (Mars, Venus) and developments related to the Hubble Telescope.
Newest articles

A universal framework for spatial biology
SpatialData is a freely accessible tool to unify and integrate data from different omics technologies accounting for spatial information, which can provide holistic insights into health and disease. Biological processes…

How complex biological processes arise
A $20 million grant from the U.S. National Science Foundation (NSF) will support the establishment and operation of the National Synthesis Center for Emergence in the Molecular and Cellular Sciences (NCEMS) at…

Airborne single-photon lidar system achieves high-resolution 3D imaging
Compact, low-power system opens doors for photon-efficient drone and satellite-based environmental monitoring and mapping. Researchers have developed a compact and lightweight single-photon airborne lidar system that can acquire high-resolution 3D…