Molecule flash mob
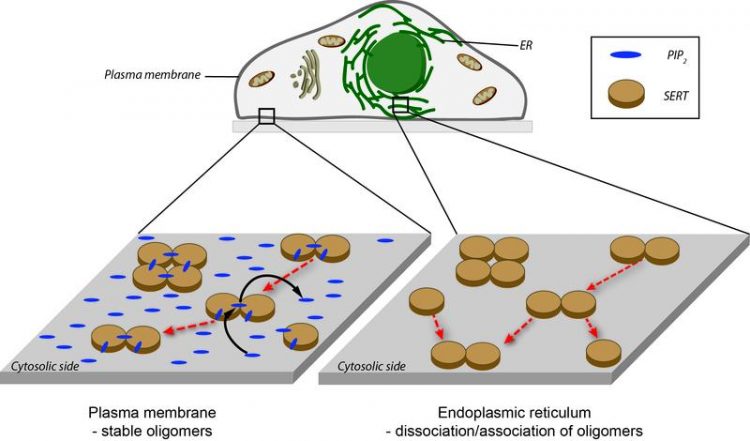
High PIP2 concentrations on the cell membrane (left) prohibit SERT oligomerisation or dissociation so the level of oligomerisation is fixed. The PIP2 concentration in the endoplasmic reticulum is very low (right). The SERT oligomerisation therefore strives for equilibrium. TU Wien
The membrane of a cell is composed of a lipid bilayer. Lipids are good chemical and electrical insulators, which are ideally suited to separating the inside of the cell from the outside of the cell. But membranes also harbour a large number of proteins, some of which regulate the controlled exchange of substances across the membrane. While the majority of proteins are able to move freely within the lipid layers, they are found in groups with surprising frequency.
The bonds between proteins may be fixed and permanent; or protein molecules may come together, split and come together again in another configuration. The research interests of the groups involved in the study, who were led by Prof. Gerhard Schütz from the Institute of Applied Physics at TU Wien and Prof. Harald Sitte from the Institute of Pharmacology at the Medical University of Vienna, concern how these interactions work, and this could shed further light on how the cell membrane and the membrane proteins embedded in it function.
Molecule tracking
Due to their size, protein molecules cannot be seen with the naked eye, which is why it is necessary to use a microscope to track them. However, the challenge lies in filtering out precisely those proteins which are of interest from the large number of other proteins in a cell.
“Together with Prof. Harald Sitte from the Medical University of Vienna, at present we are particularly interested in the serotonin transporter (SERT), a protein that is important for the uptake of the neurotransmitter serotonin in the brain. In order to be able to observe it under a microscope, it is marked with a fluorescent biomolecule, a ‘green fluorescent protein’ (GFP)’ explains Prof. Schütz. A molecular biological method is used to combine the GFP with the SERT and it then acts like a coloured balloon.
“The ‘single molecule microscopy’ method enables us to determine from the strength of the signal from the points of light observed whether the molecule in question is moving around by itself or with other molecules of the same type.”
As a biophysicist, Schütz is not only interested in the fact that the proteins move around together but also in why the two molecules stick together, in other words how the underlying interaction mechanisms work. PIP2 (Phosphatidylinositol-4,5-bisphosphate) is a central signal molecule that is predominantly found on the side of the cell membrane facing the inside of the cell. It binds perfectly to the SERT, with amazing consequences.
If only a low concentration of PIP2 is available, the oligomerisation of the SERT behaves as expected: low SERT concentrations mainly produce monomers, while high concentrations lead to the formation of oligomers. “It is not just that we are seeing a large number of oligomers, we also know that they exchange molecules with each other,” says Schütz of his research. However, if there are high levels of PIP2 available, on average oligomers that are identical in size will always be produced, regardless of the SERT concentration. “It is as though the SERT proteins were locked in a predefined arrangement.”
Exploring cluster formation
To investigate the underlying mechanisms of this amazing oligomer formation, you have to look inside the cell. One cell area – the endoplasmic reticulum – acts as the place where all membrane proteins, including SERT, are produced. This is in fact PIP2-free, meaning that SERT should have different levels of oligomerisation there, depending on the concentration of SERT. This has actually been observed too. “We assume that SERT oligomerises after it is produced in the endoplasmic reticulum, but that this process is initially reversible. It is only when the protein reaches the cell membrane that the predefined level of oligomerisation is fixed by PIP2,” says Schütz. “These observations are confirmed by specific changes to the protein structure that we inserted into the serotonin transporter,” says Sitte. “We were able to identify the position of the point mutations extremely accurately using computer models which Dr Thomas Stockner created as part of this study. The mutated SERT molecules exhibit a behaviour that makes it almost impossible for this locking to occur. And the medical relevance of our observation lies in the importance of oligomerisation for the behaviour of different psychostimulants, such as amphetamines: these can only have an effect if sufficient bonded SERT molecules are evident in the membrane.”
This study was carried out under the auspices of the Special Research Program SFB35 ‘Transmembrane Transporters in Health and Disease’ funded by the Austrian Science Fund (FWF), which mainly comprises scientists from the Medical University of Vienna. The co-author of the study and spokesperson of the SFB is the pharmacologist Harald Sitte, who, for many years, has been interested in how SERT and other transporter proteins work and how they can be modulated by psychopharmaceuticals.
Original publication:
Anderluh, A. et al. Direct PIP2 binding mediates stable oligomer formation of the serotonin transporter. Nat. Commun. 7, 14089 | DOI: 10.1038/ncomms14089 (2016)
Picture download: https://www.tuwien.ac.at/dle/pr/aktuelles/downloads/2017/flashmob
Further information:
Univ.Prof. Dipl.-Ing. Dr.techn. Gerhard Schütz
TU Wien
Institute of Applied Physics
Getreidemarkt 9, 1060 Vienna
T: +43-1-58801-13480
gerhard.schuetz@tuwien.ac.at
Univ.Prof. Dr. Harald Sitte
Medical University of Vienna
Center for Physiology and Pharmacology
Institute of Pharmacology
Währingerstraße 13a, 1040 Vienna
T: +43-1-40160-31323
harald.sitte@meduniwien.ac.at
Media Contact
All latest news from the category: Physics and Astronomy
This area deals with the fundamental laws and building blocks of nature and how they interact, the properties and the behavior of matter, and research into space and time and their structures.
innovations-report provides in-depth reports and articles on subjects such as astrophysics, laser technologies, nuclear, quantum, particle and solid-state physics, nanotechnologies, planetary research and findings (Mars, Venus) and developments related to the Hubble Telescope.
Newest articles

A universal framework for spatial biology
SpatialData is a freely accessible tool to unify and integrate data from different omics technologies accounting for spatial information, which can provide holistic insights into health and disease. Biological processes…

How complex biological processes arise
A $20 million grant from the U.S. National Science Foundation (NSF) will support the establishment and operation of the National Synthesis Center for Emergence in the Molecular and Cellular Sciences (NCEMS) at…

Airborne single-photon lidar system achieves high-resolution 3D imaging
Compact, low-power system opens doors for photon-efficient drone and satellite-based environmental monitoring and mapping. Researchers have developed a compact and lightweight single-photon airborne lidar system that can acquire high-resolution 3D…