Can material rivaling graphene be mined out of rocks? Yes, if…

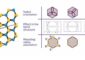
Layers of molybdenum disulfide stand better prospects of finding applications in electronics than graphene. Molybdenum disulfide occurs in nature as molybdenite, crystalline material that frequently takes the characteristic form of silver-colored hexagonal plates. (Source: FUW)
There are many signs that might prove to be the case. But physicists from the Faculty of Physics at the University of Warsaw have shown that the nature of the phenomena occurring in layered materials are still ill-understood and require further research.
Graphene has already been hailed as the future of electronics. Built of six-atom carbon rings arranged in a honeycomb-like structure, it forms extremely resilient sheets just a single atom thick. However, we do know of other materials that have a similar, layered structure. Importantly, some of them, such as molybdenum disulfide, have properties just as intriguing as those of graphene.
Researchers at the University of Warsaw, Faculty of Physics (FUW) have shown that the phenomena occurring in the crystal network of molybdenum disulfide sheets are of a slightly different nature than previously thought. A report describing the discovery, achieved in collaboration with Laboratoire National des Champs Magnétiques Intenses in Grenoble, has recently been published in Applied Physics Letters.
“It will not become possible to construct complex electronic systems consisting of individual atomic sheets until we have a sufficiently good understanding of the physics involved in the phenomena occurring within the crystal network of those materials. Our research shows, however, that research still has a long way to go in this field”, says Prof. Adam Babiński at the UW Faculty of Physics.
The simplest method of creating graphene is called exfoliation: a piece of scotch tape is first stuck to a piece of graphite, then peeled off. Among the particles that remain stuck to the tape, one can find microscopic layers of graphene. This is because graphite consists of many graphene sheets adjacent to one another. The carbon atoms within each layer are very strongly bound to one another (by covalent bonds, to which graphene owes its legendary resilience), but the individual layers are held together by significantly weaker bonds (van de Walls bonds). Ordinary scotch tape is strong enough to break the latter and to tear individual graphene sheets away from the graphite crystal.
A few years ago it was noticed that just as graphene can be obtained from graphite, sheets a single atom thick can similarly be obtained from many other crystals. This has been successfully done, for instance, with transition metals chalcogenides (sulfides, selenides, and tellurides). Layers of molybdenum disulfide (MoS2), in particular, have proven to be a very interesting material. This compound exists in nature as molybdenite, a crystal material found in rocks around the world, frequently taking the characteristic form of silver-colored hexagonal plates. For years molybdenite has been used in the manufacturing of lubricants and metal alloys. Like in the case of graphite, the properties of single-atom sheets of MoS2 long went unnoticed.
From the standpoint of applications in electronics, molybdenum disulfide sheets exhibit a significant advantage over graphene: they have an energy gap, an energy range within which no electron states can exist. By applying electric field, the material can be switched between a state that conducts electricity and one that behaves like an insulator. By current calculations, a switched-off molybdenum disulfide transistor would consume even as little as several hundred thousand times less energy than a silicon transistor. Graphene, on the other hand, has no energy gap and transistors made of graphene cannot be fully switched off.
Valuable information about a crystal's structure and phenomena occurring within it can be obtained by analyzing how light gets scattered within the material. Photons of a given energy are usually absorbed by the atoms and molecules of the material, then reemitted at the same energy. In the spectrum of the scattered light one can then see a distinctive peak, corresponding to that energy. It turns out, however, that one out of many millions of photons is able to use some of its energy otherwise, for instance to alter the vibration or circulation of a molecule. The reverse situation also sometimes occurs: a photon may take away some of the energy of a molecule, and so its own energy slightly increases. In this situation, known as Raman scattering, two smaller peaks are observed to either side of the main peak.
The scientists at the UW Faculty of Physics analyzed the Raman spectra of molybdenum disulfide carrying on low-temperature microscopic measurements. The higher sensitivity of the equipment and detailed analysis methods enabled the team to propose a more precise model of the phenomena occurring in the crystal network of molybdenum disulfide.
“In the case of single-layer materials, the shape of the Raman lines has previously been explained in terms of phenomena involving certain characteristic vibrations of the crystal network. We have shown for molybdenum disulfide sheets that the effects ascribed to those vibrations must actually, at least in part, be due to other network vibrations not previously taken into account”, explains Katarzyna Gołasa, a doctorate student at the UW Faculty of Physics.
The presence of the new type of vibration in single-sheet materials has an impact on how electrons behave. As a consequence, these materials must have somewhat different electronic properties than previously anticipated.
“Graphene was the first. Its unique characteristics have triggered a considerable, still-growing interest among scientists and also from industry. However, we must not forget about other single-layer materials. If we study them well, they may prove to be better than graphene for many applications”, Prof. Babiński says.
Physics and Astronomy first appeared at the University of Warsaw in 1816, under the then Faculty of Philosophy. In 1825 the Astronomical Observatory was established. Currently, the Faculty of Physics' Institutes include Experimental Physics, Theoretical Physics, Geophysics, Department of Mathematical Methods and an Astronomical Observatory. Research covers almost all areas of modern physics, on scales from the quantum to the cosmological. The Faculty's research and teaching staff includes ca. 200 university teachers, of which near 80 are employees with the title of professor. The Faculty of Physics, University of Warsaw, is attended by ca. 1000 students and more than 140 doctoral students.
CONTACTS:
Prof. Adam Babiński
Faculty of Physics, University of Warsaw
tel. +48 22 5532374, +48 22 5532266
email: adam.babinski@fuw.edu.pl
RELATED LINKS:
http://www.fuw.edu.pl/ Faculty of Physics at the University of Warsaw website.
http://www.fuw.edu.pl/informacje-prasowe.html Press Office for the Faculty of Physics at the University of Warsaw.
Media Contact
More Information:
http://www.fuw.edu.plAll latest news from the category: Physics and Astronomy
This area deals with the fundamental laws and building blocks of nature and how they interact, the properties and the behavior of matter, and research into space and time and their structures.
innovations-report provides in-depth reports and articles on subjects such as astrophysics, laser technologies, nuclear, quantum, particle and solid-state physics, nanotechnologies, planetary research and findings (Mars, Venus) and developments related to the Hubble Telescope.
Newest articles

A universal framework for spatial biology
SpatialData is a freely accessible tool to unify and integrate data from different omics technologies accounting for spatial information, which can provide holistic insights into health and disease. Biological processes…

How complex biological processes arise
A $20 million grant from the U.S. National Science Foundation (NSF) will support the establishment and operation of the National Synthesis Center for Emergence in the Molecular and Cellular Sciences (NCEMS) at…

Airborne single-photon lidar system achieves high-resolution 3D imaging
Compact, low-power system opens doors for photon-efficient drone and satellite-based environmental monitoring and mapping. Researchers have developed a compact and lightweight single-photon airborne lidar system that can acquire high-resolution 3D…