New target: Researchers identify pancreatic cancer resistance mechanism

This image depicts Haoqiang Ying, Ph.D. Credit: MD Anderson Cancer Center
Pancreatic cancer tumors addicted to mutant Kras signaling for their growth and progression have a ready-made substitute to tap if they're ever forced to go cold-turkey on the mutant oncogene, scientists at The University of Texas MD Anderson Cancer Center report in the journal Cell.
When researchers dialed up mutant Kras to spur pancreatic cancer growth in mice, and then shut it down, a group of recurrent tumors grew back independently of mutant Kras, reliant on a different oncogene.
“There's a great deal of effort under way trying to find ways to target Kras or some of the downstream targets that it activates,” said co-lead author Haoqiang Ying, Ph.D., assistant professor of Molecular and Cellular Oncology. “It's important to understand how Kras-dependent tumors might evolve in response to targeted therapy.”
The team found that some recurrent tumors were completely independent of mutant Kras and instead relied on signaling through another known oncogene called Yap1.
In addition to discovering a resistance mechanism for Kras-dependent tumors, the team found tumors dependent on Yap1 resemble a specific type of pancreatic tumor that provides a poor prognosis for patients who have it.
“Pancreas cancer remains an intractable disease with limited therapeutic options,” said senior author Ron DePinho, M.D., president and professor of Cancer Biology. “Identifying and validating key targets in faithful model systems represents a critical first step in ultimately providing our patients with meaningful therapies.”
Pancreatic ductal adenocarcinoma is one of the most lethal of cancers, with only 6.7 percent of patients surviving for five years. An estimated 46,420 new cases will be diagnosed in 2014, and approximately 39,590 people will die of the disease, according to the National Cancer Institute.
Mutations that activate Kras are present in the majority of human pancreatic cancer cases. Mouse model experiments, including work by DePinho, Ying and colleagues, have demonstrated critical roles for those mutations in tumor initiation and progression.
Turning Kras on and off
To conduct the research, the team used a genetically engineered mouse model of inducible Kras-dependent pancreatic cancer developed in DePinho's lab when he was at the Dana-Farber Cancer Institute in Boston. The mice have a version of Kras that can be turned on by treating them with doxycycline, an antibiotic, and only develop pancreatic cancer when this occurs. Tumors develop swiftly and then begin to regress after 24 hours of cessation of doxycycline treatment.
Within three weeks of doxycycline cessation, tumors regressed completely in all 28 mice. Then, 20 of the mice had a recurrence between nine and 47 weeks later. The recurrent tumors had characteristics of aggressive disease, including spread to the lung or liver in 15 of the mice.
Half of the recurrent tumors had re-expression of the inducible Kras transgene, while the other half had no sign of the oncogene or of activation of its related molecular pathways.
If not Kras, then Yap1
To identify the driving molecular mechanism for the non-Kras recurrent tumors, the team conducted an analysis to identify copy number variations of genes in the tumors.
“The only gene amplified was Yap1, which made sense, because it's a known oncogene,” said co-lead author Avnish Kapoor, Ph.D., a postdoctoral fellow in Genomic Medicine who conducted the analysis.
Functional studies conducted by Wantong Yao, Ph.D., also a postdoctoral fellow in Genomic Medicine, confirmed the finding.
Yap1-amplified recurrent tumors shrank when Yap1 expression was knocked down using RNA interference. Yap1 expression in the Kras-dependent mice stifled tumor regression and supported tumor growth after doxycycline withdrawal
Mapping the Yap1 pathway
Yap1 is involved in gene transcription – the activation of genes by proteins that bind to the gene's DNA in its promoter region – but does not itself bind to DNA. As a co-activator, it works through other transcription factors.
Enabled by genome-scale unbiased and molecular analyses, the researchers found that Yap1 forms a complex with Tead2, one of its known partners, and then works with E2F, another transcription factor. Together, they activate a cell cycle and DNA replication program to support tumor survival and growth.
“With Kras turned off, Yap1 can recreate this transcription program involving cell cycle and DNA replication machinery that is normally controlled by Kras,” Yao said.
Yap1 and human tumors Recent classification of pancreatic cancer based on gene transcription profiles identified a subtype that is not dependent on Kras. These so-called quasimesenchymal tumors have a poor prognosis.
The team confirmed that Yap1 is highly expressed in these tumor cell lines and that knocking down Yap1 suppressed proliferation of these cells.
Yap1 is known to be involved in cell proliferation, a cellular conversion known as epithelial-to-mesenchymal transition, cancer invasion and metastasis and has been found amplified in liver, oral squamous cell and esophageal cancers as well as in medulloblastoma.
While Yap1 drives tumor recurrence and progression, Kapoor said evidence shows that it is insufficient to drive initial formation of pancreatic cancer.
Yap1, like Kras, is not presently targetable with a drug. The researchers note that small molecules targeting Yap1 have stalled liver cancer progression in a mouse model and might eventually prove to work in Yap1-dependent cancer. Clinical trials of this approach are years away.
Future studies by the team include understanding Yap1's role in quasimesenchymal pancreatic cancer and exploring potential signaling connections between Kras and Yap1.
They'll also look for additional mechanisms for tumors to escape Kras addiction, such as activation of other growth factor pathways, Ying said. “Characterization of these pathways may identify other potential therapeutic targets for this dreaded disease.”
Co-authors with Ying, Kapoor, Yao and DePinho are: Sujun Hua, Alison Liewen, and Qiuyun
Wang, Chang-Jiun Wu, Ramsey Al-Khalil, Shan Jiang, Hongai Xia, Eliot Fletcher-Sananikone, Carol Lim, Gillian I. Horwitz, Andrea Viale, Piergiorgio Pettazzoni, Nora Sanchez, Baoli Hu, Y. Alan Wang, Giulio Draetta, and Lynda Chin, all of Cancer Genomics; Qing Chang, and Yao, Ying, Viale, Pettazzoni, Sanchez and are in Molecular and Cellular Oncology; Yi Zhong, Alexei Protopopov, Jianhua Zhang, Timothy Heffernan, and Draetta are with the Institute for Applied Cancer Science; Huamin Wang of Pathology; Randy Johnson of Biochemistry and Molecular Biology; Anguraj Sadanandam of the Institute of Cancer Research in the United Kingdom and the Swiss Institute for Experimental Cancer Research, Lausanne, Switzerland; and Gerald Chu, of Pathology, Brigham and Women's Hospital, Boston.
This research was funded by grants from the National Cancer Institute of the National Institutes of Health (CA084313, P01CA117969, R56DK094865), a University of Texas Star Award; The NIH National Institute for Health Research; a U.S. Department of Defense Discovery Award; the Biomedical Research Centre for Cancer; the American-Italian Cancer Foundation; MD Anderson's Sheikh Ahmed Bin Zayed Al Nahyan Center for Pancreatic Cancer Research; and postdoctoral fellowships from the Jane Coffin Childs Fund, the Damon Runyon Cancer Research Foundation and MD Anderson's Translational Research in Multi-Disciplinary Program (TRIUMPH).
Media Contact
All latest news from the category: Health and Medicine
This subject area encompasses research and studies in the field of human medicine.
Among the wide-ranging list of topics covered here are anesthesiology, anatomy, surgery, human genetics, hygiene and environmental medicine, internal medicine, neurology, pharmacology, physiology, urology and dental medicine.
Newest articles

Why getting in touch with our ‘gerbil brain’ could help machines listen better
Macquarie University researchers have debunked a 75-year-old theory about how humans determine where sounds are coming from, and it could unlock the secret to creating a next generation of more…
Attosecond core-level spectroscopy reveals real-time molecular dynamics
Chemical reactions are complex mechanisms. Many different dynamical processes are involved, affecting both the electrons and the nucleus of the present atoms. Very often the strongly coupled electron and nuclear…
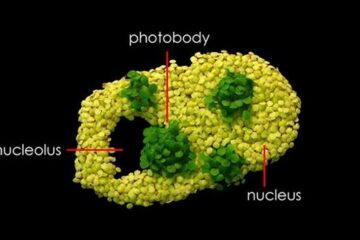
Free-forming organelles help plants adapt to climate change
Scientists uncover how plants “see” shades of light, temperature. Plants’ ability to sense light and temperature, and their ability to adapt to climate change, hinges on free-forming structures in their…