Flu antibody’s 'one-handed grab' may boost effort toward universal vaccine, new therapies
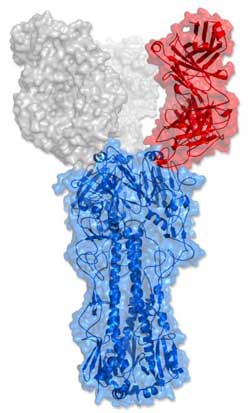
To counteract a broad range of dangerous flu viruses, the unique C05 antibody (red) binds to influenza’s hemagglutinin protein (blue) in an unusual way.<br><br>Credit: Wilson lab, The Scripps Research Institute<br>
Scientists from The Scripps Research Institute and Sea Lane Biotechnologies have solved the co-crystal structure of a human antibody that can neutralize influenza viruses in a unique way.
The antibody recognizes the crucial structure that flu viruses use to attach to host cells, even though previously this structure had been thought too small for an antibody to grab effectively. The immune protein manages to hit this precise spot by using just a small part of its target-grabbing apparatus. In so doing, it can neutralize a broad range of dangerous flu viruses.
“This highly focused binding to the receptor binding site using only a single loop on the antibody has never been seen before, and it's really fascinating; it gives us some good ideas about designs for vaccines and therapies,” said Ian A. Wilson, the Hansen Professor of Structural Biology at Scripps Research. Wilson was senior investigator of the study along with Ramesh R. Bhatt of Sea Lane Biotechnologies. The report appears online ahead of print on September 16, 2012, in the journal Nature.
The Power of Large Numbers
Sea Lane Biotechnologies, advised by Richard Lerner, the Lita Annenberg Hazen Professor of Immunochemistry at Scripps Research, began by collecting bone marrow from patients who had been exposed to certain key strains of flu. Because the bone marrow is a “fossil record” of all the antibodies a person has ever made, those at Sea Lane felt confident that the antibodies they were looking for would be there. Sea Lane's efforts even went as far as to include advertised appeals on Craigslist for individuals who had certain strains of the flu. Using the internationally and locally collected bone marrow, Sea Lane generated a “comprehensive flu library” of billions of antibodies.
Sea Lane Biotechnologies scientists, led by Bhatt, isolated the unusual new antibody, which they dubbed C05, by screening this enormous library for antibodies that could bind to proteins from a variety of influenza A viruses—the most dangerous family of flu viruses.
C05 also protected cells in the lab dish from infection by these viruses. In mice, relatively low doses of C05 prevented infections despite influenza A exposures that would have been lethal. The antibody worked as a therapy, too, rescuing 100 percent of mice when administered up to three days after a flu infection had begun.
An Unusual Grip for a Tricky Target
Further tests revealed a curious property of C05. Almost uniquely among broadly neutralizing antibodies against influenza A, it specifically recognizes and blocks the part of the flu virus that mediates viral attachment to host cells. Known as the “receptor binding site” (RBS), this viral site is located on the heads of viral hemagglutinins, spiky structures of sugar and protein that coat the viral envelope.
The RBS is a key functional site on flu viruses and is relatively exposed to the immune system compared to other viral regions. It would be an ideal target for antibodies, except that it is quite small compared to an antibody's usual grip area.
An antibody binds targets using two arm-like structures, each of which has six protein fingers or loops. To get a good grip on the flu RBS with some or all these loops, a typical antibody has to grab not only the RBS, but also some of the surrounding structures in the head, which vary from one flu strain to another. Thus, an antibody that does get a firm grip on this region for one flu strain generally will lose that grip once the strain mutates. “This is why universal flu vaccine strategies in recent years have been focused more on the hemagglutinin stem than on the head,” noted Damian C. Ekiert, formerly of Wilson's laboratory at Scripps Research and now of University of California, San Francisco. Ekiert was first author of the paper with Arun K. Kashyap of Sea Lane Biotechnologies.
Wilson's laboratory specializes in the use of X-ray crystallography and other techniques to determine precisely where and how such antibodies bind to their viral targets. Using these methods, his team found that C05 effectively avoids grabbing the hypervariable regions around the flu RBS. Instead it uses a single elongated protein loop to reach in and make a “one-handed”—or “one-fingered”—grab of the RBS itself. The antibody apparently works best when two of these active loops, one on each arm, grab two viral RBSs on separate hemagglutinins. “It looks like these antibodies need to cross-link two hemagglutinins to have maximum effect,” said Wilson.
Potentially Useful
The RBS has such an important function that it does not change much from strain to strain—and thus C05 can neutralize a broad range of dangerous influenza A viruses, including H1, H2, H3, and H9 subtypes.
The fact that C05 can make such a precision grab suggests that it and perhaps even more potent antibodies could contribute to antibody-based therapies for severe influenza infections. A universal flu vaccine also might be significantly more effective if it could be designed to elicit such antibodies in people.
“If we can figure out how to induce this sort of antibody in a vaccine, we would have something that's very useful,” said Lawrence Horowitz, CEO of Sea Lane Biotechnologies.
In addition to Wilson, Bhatt, Ekiert, Kashyap, and Horowitz, authors of the paper, “Neutralization of Influenza A viruses by insertion of a single antibody loop into the receptor binding site,” included Michael A. Dillon, Ryann E. O'Neil, Aleksandr M. Faynboym, and Michael Horowitz of Sea Lane; John Steele and Peter Palese of the Mount Sinai School of Medicine; Adam Rubrum and Richard Webby of St. Jude Children's Research Hospital of Memphis, Tennessee; and Gira Bhabha, Reza Khayat, Jeong Hyun Lee, and Andrew B. Ward of Scripps Research.
The study was funded in part by grants from the National Institutes of Health (P01 AI058113 and GM080209) and the Skaggs Institute for Chemical Biology at Scripps Research.
About The Scripps Research Institute
The Scripps Research Institute is one of the world's largest independent, not-for-profit organizations focusing on research in the biomedical sciences. Over the past decades, Scripps Research has developed a lengthy track record of major contributions to science and health, including laying the foundation for new treatments for cancer, rheumatoid arthritis, hemophilia, and other diseases. The institute employs about 3,000 people on its campuses in La Jolla, CA, and Jupiter, FL, where its renowned scientists—including three Nobel laureates—work toward their next discoveries. The institute's graduate program, which awards Ph.D. degrees in biology and chemistry, ranks among the top ten of its kind in the nation. For more information, see www.scripps.edu.
About Sea Lane Biotechnologies, LLC
Sea Lane Biotechnologies, LLC is focused on innovating biotherapeutic discovery with transformative technologies and platforms that are necessary to meet contemporary pharmaceutical biologic portfolio requirements. Founded in 2005 in the Silicon Valley, the company is dedicated to the discovery and development of biotherapeutic drugs based upon a novel class of proteins called Surrobodies™ and world-class antibodies for the treatment of serious human diseases, such as cancer and viral infection. Sea Lane currently has several proprietary product candidates in pre-clinical testing.
Media Contact
More Information:
http://www.scripps.eduAll latest news from the category: Health and Medicine
This subject area encompasses research and studies in the field of human medicine.
Among the wide-ranging list of topics covered here are anesthesiology, anatomy, surgery, human genetics, hygiene and environmental medicine, internal medicine, neurology, pharmacology, physiology, urology and dental medicine.
Newest articles

A universal framework for spatial biology
SpatialData is a freely accessible tool to unify and integrate data from different omics technologies accounting for spatial information, which can provide holistic insights into health and disease. Biological processes…

How complex biological processes arise
A $20 million grant from the U.S. National Science Foundation (NSF) will support the establishment and operation of the National Synthesis Center for Emergence in the Molecular and Cellular Sciences (NCEMS) at…

Airborne single-photon lidar system achieves high-resolution 3D imaging
Compact, low-power system opens doors for photon-efficient drone and satellite-based environmental monitoring and mapping. Researchers have developed a compact and lightweight single-photon airborne lidar system that can acquire high-resolution 3D…





















