IASLC: Adjunctive use of 3-D imaging system increases utility of CT lung cancer screening

Amsterdam, the Netherlands and Phoenix, AZ, USA – July 4, 2011 – VisionGate, Inc., a company developing a revolutionary non-invasive test for the early detection of lung cancer, today announced that it will present data showing how adjunctive use of its LuCED™ test can improve the utility of low dose x-ray computed tomography (CT) screening for the early detection of lung cancer in high risk individuals.
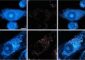
LuCED uses VisionGate's revolutionary automated 3D cell imaging platform, the Cell-CT™, which generates high-resolution 3D biosignatures from intact cells using a sputum sample. The data will be presented at the International Association for the Study of Lung Cancer's 14th World Conference on Lung Cancer on July 7, 2011 from 9:30am-12:30pm CEST.
The proposed first use of LuCED as an adjunct to CT screening reflects the results of the National Cancer Institute's (NCI) landmark National Lung Screening Trial (NLST) of more than 53,000 current and former heavy smokers, which showed that low-dose helical CT screening reduced lung cancer deaths by 20% compared to standard chest x-rays. These dramatic results were first presented last November and were expanded this week in the June 29, 2011 online edition of the New England Journal of Medicine.
The NLST findings are generating widespread interest in the broad use of CT screening as a way to reduce lung cancer deaths in high-risk populations. However, the utility of the approach is hampered by the high rate of false positive results seen in the study—according to the NCI, more than 96% of the positive results from low-dose CT screening over three rounds of testing turned out to be false positive findings, representing almost a quarter (23.3%) of the overall test results. These false positive test results require follow-up care that results in unnecessary invasive procedures for many patients and significantly higher costs for the healthcare system as a whole, potentially jeopardizing the feasibility of implementing mass screening programs for the millions of individuals at high risk for lung cancer.
In its presentation, VisionGate shows how LuCED harnesses the power of 3D imaging to accurately detect cancer cells when present in sputum samples from individuals at high risk of lung cancer. The presentation outlines patient management protocols for the non-invasive and efficient use of LuCED as an adjunct to CT screening to confirm which cases are true positives and to lower significantly the number of cases which are false positives.
Dr. Claudia Henschke, a lung cancer researcher at the Biodesign Institute at Arizona State University who is also a practicing physician at Mount Sinai Medical Center in New York City, serves as a leader of the International Early Lung Cancer Action Program. She is co-author of a pioneering study of the benefits of CT scans for lung cancer that was published in the New England Journal of Medicine in 2006.
Dr. Henschke noted, “Today, almost all patients diagnosed with lung cancer in the U.S. die. The NLST study results released last November confirmed our initial findings showing that CT scans can find lung cancers in their earliest stage, when up to 92% can be cured. VisionGate's LuCED technology has demonstrated promise as an approach that may increase the feasibility of implementing widespread screening of high risk individuals, initially being used as an adjunct to improve the accuracy of CT scan results, with the potential to be used as a primary screening tool if additional trials are successful. We look forward to the opportunity to contribute to assessments of its potential utility in helping to reduce the death rate from this lethal cancer that affects so many people around the globe.”
LuCED uses the company's breakthrough automated Cell-CT platform to produce detailed 3D images of the cells contained in sputum samples, which the system analyzes to identify key features associated with potential malignancy. The analysis yields a score that indicates whether or not cancer cells are present in the sample. The Cell-CT system produces strikingly clear and comprehensive 3D images of the cells, enabling extremely accurate classifications. VisionGate researchers estimate that LuCED will be able to achieve specificity of 99% and sensitivity of 75% in actual use.
“The dramatic results of the NLST study provide us with a valuable initial indication for our LuCED test,” commented Scarlett Spring, president of VisionGate. “By combining the high accuracy and cost effectiveness of our non-invasive LuCED diagnostic with the proven ability of CT screening to reduce lung cancer deaths, we hope to make mass screening feasible and affordable. We believe that we have developed an efficient clinical and regulatory strategy for our proposed use of LuCED to manage the false positive results from CT screening, and we look forward to advancing this program in the coming months.”
Prior to the development of LuCED, a number of studies had confirmed that analyzing cells in sputum could potentially be useful in the early detection of lung cancer, showing that 85% of sputum samples from individuals with lung cancer (the samples were taken over three days and pooled) actually contained cancer cells. However, the typically poor sensitivity of raw sputum analysis and the labor intensive nature of the process made it impracticable for widespread use in lung cancer screening. The Cell-CT platform's 3D technology, along with its advanced automated 3D image analysis algorithms, for the first time make a cell-based sputum analysis approach both logistically feasible and cost effective.
VisionGate's poster (P4.007), “The LuCED Test for Detection of Early Lung Cancer: A Criterion to Complete the Test with High Sensitivity,” will be presented by Michael Meyer on July 7, 2011 from 9:30am-12:30pm CEST.
The IASLC's 14th World Conference on Lung Cancer is being held in Amsterdam, the Netherlands from July 3-July 7, 2011. For more information, visit www.2011worldlungcancer.org/
About the International Association for the Study of Lung Cancer (IASLC)
Founded in 1972, the International Association for the Study of Lung Cancer (IASLC) is an international organization of nearly 3,000 lung cancer specialists spanning 80 countries. IASLC members work towards developing and promoting the study of etiology, epidemiology, prevention, diagnosis, treatment and all other aspects of lung cancer and other thoracic malignancies. IASLC's mission is to enhance the understanding and education of lung cancer to scientists, members of the medical community and the public. In addition to the biannual meeting, the IASLC publishes the Journal of Thoracic Oncology, a prized resource for medical specialists and scientists who focus on the detection, prevention, diagnosis and treatment of lung cancer.
About VisionGate
VisionGate, Inc. is developing a revolutionary non-invasive test for the early detection of lung cancer, using its automated 3D cell imaging platform, the Cell-CT™, which generates high-resolution 3D biosignatures from intact cells using a sputum sample. The company's LuCED™ test is initially being developed for adjunctive use with low dose x-ray computed tomography (CT) screening for the early detection of lung cancer in high risk individuals. Adjunctive use of LuCED to better manage the high rate of false positive results in CT screening could increase the utility and cost effectiveness of the approach, which has been shown to decrease lung cancer deaths in former and current smokers. For more information, visit www.visiongate3D.com.
Media Contact
More Information:
http://www.biocompartners.comAll latest news from the category: Medical Engineering
The development of medical equipment, products and technical procedures is characterized by high research and development costs in a variety of fields related to the study of human medicine.
innovations-report provides informative and stimulating reports and articles on topics ranging from imaging processes, cell and tissue techniques, optical techniques, implants, orthopedic aids, clinical and medical office equipment, dialysis systems and x-ray/radiation monitoring devices to endoscopy, ultrasound, surgical techniques, and dental materials.
Newest articles

A universal framework for spatial biology
SpatialData is a freely accessible tool to unify and integrate data from different omics technologies accounting for spatial information, which can provide holistic insights into health and disease. Biological processes…

How complex biological processes arise
A $20 million grant from the U.S. National Science Foundation (NSF) will support the establishment and operation of the National Synthesis Center for Emergence in the Molecular and Cellular Sciences (NCEMS) at…

Airborne single-photon lidar system achieves high-resolution 3D imaging
Compact, low-power system opens doors for photon-efficient drone and satellite-based environmental monitoring and mapping. Researchers have developed a compact and lightweight single-photon airborne lidar system that can acquire high-resolution 3D…