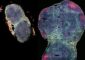
Researchers Design Alzheimer’s Antibodies

The process is reported in the Dec. 5 Early Edition of the journal Proceedings of the National Academy of Sciences (PNAS). The process, outlined in the paper, titled “Structure-based design of conformation- and sequence-specific antibodies against amyloid â,” could be used as a tool to understand complex disease pathology and develop new antibody-based drugs in the future.
Antibodies are large proteins produced by the immune system to combat infection and disease. They are comprised of a large Y-shaped protein topped with small peptide loops. These loops bind to harmful invaders in the body, such as a viruses or bacteria. Once an antibody is bound to its target, the immune system sends cells to destroy the invader. Finding the right antibody can determine the difference between death and recovery.
Scientists have long sought methods for designing antibodies to combat specific ailments. However, the incredible complexity of designing antibodies that only attached to a target molecule of interest has prevented scientists from realizing this ambitious goal.
When trying to design an antibody, the arrangement and sequence of the antibody loops is of utmost importance. Only a very specific combination of antibody loops will bind to and neutralize each target. And with billions of different possible loop arrangements and sequences, it is seemingly impossible to predict which antibody loops will bind to a specific target molecule.
The new antibody design process was used to create antibodies that target a devastating molecule in the body: the Alzheimer’s protein. The research, which was led by Assistant Professor of Chemical and Biological Engineering Peter Tessier, uses the same molecular interactions that cause the Alzheimer’s proteins to stick together and form the toxic particles that are a hallmark of the disease.
“We are actually exploiting the same protein interactions that cause the disease in the brain to mediate binding of antibodies to toxic Alzheimer’s protein particles,” Tessier said.
Alzheimer’s disease is due to a specific protein – the Alzheimer’s protein – sticking together to form protein particles. These particles then damage the normal, healthy functions of the brain. The formation of similar toxic protein particles is central to diseases such as Parkinson’s and mad cow disease.
Importantly, the new Alzheimer’s antibodies developed by Tessier and his colleagues only latched on to the harmful clumped proteins and not the harmless monomers or single peptides that are not associated with disease.
Tessier and his colleagues see the potential for their technique being used to target and better understand similar types of protein particles in disorders such as Parkinson’s disease.
“By binding to specific portions of the toxic protein, we could test hypotheses about how to prevent or reverse cellular toxicity linked to Alzheimer’s disease,” Tessier said.
In the long term, as scientists learn more about methods to deliver drugs into the extremely well-protected brain tissue, the new antibody research may also help to develop new drugs to combat disorders such as Alzheimer’s disease.
The research was funded by the Alzheimer’s Association, the National Science Foundation (NSF), and the Pew Charitable Trust.
Tessier was joined in the research by Rensselaer graduate students Joseph Perchiacca (co-first author), Ali Reza Ladiwala (co-first author), and Moumita Bhattacharya.
Media Contact
More Information:
http://www.rpi.eduAll latest news from the category: Life Sciences and Chemistry
Articles and reports from the Life Sciences and chemistry area deal with applied and basic research into modern biology, chemistry and human medicine.
Valuable information can be found on a range of life sciences fields including bacteriology, biochemistry, bionics, bioinformatics, biophysics, biotechnology, genetics, geobotany, human biology, marine biology, microbiology, molecular biology, cellular biology, zoology, bioinorganic chemistry, microchemistry and environmental chemistry.
Newest articles
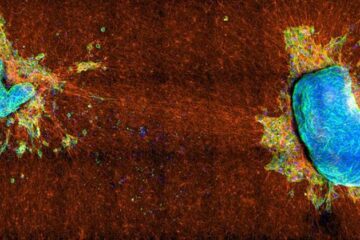
Study sheds light on cancer cell ‘tug-of-war’
How cancer cells tug against each other determines whether they can migrate elsewhere in the body. Understanding how cancerous cells spread from a primary tumor is important for any number…

Latest generation of self-dissolving stents
Magnesium implants support coronary arteries and keep them open. Constricted coronary arteries harbor dangers: Because the heart is not supplied with blood properly, this can lead to pain, cardiac arrhythmia,…

Stopping blood cancer
The MHH joint project TARGET-MPN is investigating why the disease persists and progresses in malignant bone marrow diseases from the group of myeloproliferative neoplasms despite targeted treatment. Haematopoietic stem cells…