Gold nanoparticles give an edge in recycling CO2

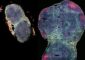
Less is more ... to a point<br> Gold nanoparticles make better catalysts for CO2 recycling than bulk gold metal. Size is crucial though, since edges produce more desired results than corners (red points, above). Nanoparticles of 8 nm appear to have a better edge-to-corner ratio than 4 nm, 6 nm, or 10 nm nanoparticles. Credit: Sun lab/Brown University <br>
Researchers from Brown have shown that finely tuned gold nanoparticles can do the job. The key is maximizing the particles’ long edges, which are the active sites for the reaction.
By tuning gold nanoparticles to just the right size, researchers from Brown University have developed a catalyst that selectively converts carbon dioxide (CO2) to carbon monoxide (CO), an active carbon molecule that can be used to make alternative fuels and commodity chemicals.
“Our study shows potential of carefully designed gold nanoparticles to recycle CO2 into useful forms of carbon,” said Shouheng Sun, professor of chemistry and one of the study’s senior authors. “The work we’ve done here is preliminary, but we think there’s great potential for this technology to be scaled up for commercial applications.”
The findings are published in the Journal of the American Chemical Society.
The idea of recycling CO2 — a greenhouse gas the planet current has in excess — is enticing, but there are obstacles. CO2 is an extremely stable molecule that must be reduced to an active form like CO to make it useful. CO is used to make synthetic natural gas, methanol, and other alternative fuels.
Converting CO2 to CO isn’t easy. Prior research has shown that catalysts made of gold foil are active for this conversion, but they don’t do the job efficiently. The gold tends to react both with the CO2 and with the water in which the CO2 is dissolved, creating hydrogen byproduct rather than the desired CO.
The Brown experimental group, led by Sun and Wenlei Zhu, a graduate student in Sun’s group, wanted to see if shrinking the gold down to nanoparticles might make it more selective for CO2. They found that the nanoparticles were indeed more selective, but that the exact size of those particles was important. Eight nanometer particles had the best selectivity, achieving a 90-percent rate of conversion from CO2 to CO. Other sizes the team tested — four, six, and 10 nanometers — didn’t perform nearly as well.
“At first, that result was confusing,” said Andrew Peterson, professor of engineering and also a senior author on the paper. “As we made the particles smaller we got more activity, but when we went smaller than eight nanometers, we got less activity.”
To understand what was happening, Peterson and postdoctoral researcher Ronald Michalsky used a modeling method called density functional theory. They were able to show that the shapes of the particles at different sizes influenced their catalytic properties.
“When you take a sphere and you reduce it to smaller and smaller sizes, you tend to get many more irregular features — flat surfaces, edges and corners,” Peterson said. “What we were able to figure out is that the most active sites for converting CO2 to CO are the edge sites, while the corner sites predominantly give the by-product, which is hydrogen. So as you shrink these particles down, you’ll hit a point where you start to optimize the activity because you have a high number of these edge sites but still a low number of these corner sites. But if you go too small, the edges start to shrink and you’re left with just corners.”
Now that they understand exactly what part of the catalyst is active, the researchers are working to further optimize the particles. “There’s still a lot of room for improvement,” Peterson said. “We’re working on new particles that maximize these active sites.”
The researchers believe these findings could be an important new avenue for recycling CO2 on a commercial scale.
“Because we’re using nanoparticles, we’re using a lot less gold than in a bulk metal catalyst,” Sun said. “That lowers the cost for making such a catalyst and gives the potential to scale up.”
The work was funded by a National Science Foundation grant to the Brown-Yale Center for Chemical Innovation (CCI), which looks for ways to use CO2 as a sustainable feedstock for large-scale commodity chemicals. Other authors on the paper were Önder Metin, Haifeng Lv, Shaojun Guo, Christopher Wright, and Xiaolian Sun.
Editors: Brown University has a fiber link television studio available for domestic and international live and taped interviews, and maintains an ISDN line for radio interviews. For more information, call (401) 863-2476.
Media Contact
More Information:
http://www.brown.eduAll latest news from the category: Life Sciences and Chemistry
Articles and reports from the Life Sciences and chemistry area deal with applied and basic research into modern biology, chemistry and human medicine.
Valuable information can be found on a range of life sciences fields including bacteriology, biochemistry, bionics, bioinformatics, biophysics, biotechnology, genetics, geobotany, human biology, marine biology, microbiology, molecular biology, cellular biology, zoology, bioinorganic chemistry, microchemistry and environmental chemistry.
Newest articles
Red light therapy for repairing spinal cord injury passes milestone
Patients with spinal cord injury (SCI) could benefit from a future treatment to repair nerve connections using red and near-infrared light. The method, invented by scientists at the University of…
Insect research is revolutionized by technology
New technologies can revolutionise insect research and environmental monitoring. By using DNA, images, sounds and flight patterns analysed by AI, it’s possible to gain new insights into the world of…

X-ray satellite XMM-newton sees ‘space clover’ in a new light
Astronomers have discovered enormous circular radio features of unknown origin around some galaxies. Now, new observations of one dubbed the Cloverleaf suggest it was created by clashing groups of galaxies….