The flexible tail of the prion protein poisons brain cells

Neuropathologists from the University of Zurich and University Hospital Zurich have now shown that it is the flexible tail of the prion protein that triggers cell death. These findings have far-reaching consequences: only those antibodies that target the tail of the prion protein are suitable as potential drugs for combating prion diseases.
Prion proteins are the infectious pathogens that cause Mad Cow Disease and Creutzfeldt-Jakob disease. They occur when a normal prion protein becomes deformed and clumped. The naturally occurring prion protein is harmless and can be found in most organisms. In humans, it is found in our brain cell membrane. By contrast, the abnormally deformed prion protein is poisonous for the brain cells.
Adriano Aguzzi, Professor of Neuropathology at the University of Zurich and University Hospital Zurich, has spent many years exploring why this deformation is poisonous. Aguzzi’s team has now discovered that the prion protein has a kind of «switch» that controls its toxicity. This switch covers a tiny area on the surface of the protein. If another molecule, for example an antibody, touches this switch, a lethal mechanism is triggered that can lead to very fast cell death.
Flexible tail induces cell death
In the current edition of «Nature», the scientists demonstrate that the prion protein molecule comprises two functionally distinct parts: a globular domain, which is tethered to the cell membrane, and a long and unstructured tail. Under normal conditions, this tail is very important in order to maintain the functioning of nerve cells. By contrast, in the case of a prion infection the pathogenic prion protein interacts with the globular part and the tail causes cell death – this is the hypothesis put forward by the researchers.
Aguzzi and his team tested this by generating mimetic antibodies in tissue sections from the cerebellum of mice which have a similar toxicity to that of a prion infection. The researchers found that these antibodies tripped the switch of the prion protein. «Prion proteins with a trimmed version of the flexible tail can, however, no longer damage the brain cells, even if their switch has been recognized by antibodies», explains Adriano Aguzzi. «This flexible tail is responsible for causing cell death.» If the tail is bound and made inaccessible using a further antibody, activation of the switch can likewise no longer trigger cell death.
«Our discovery has far-reaching consequences for understanding prion diseases», says Aguzzi. The findings reveal that only those antibodies that target the prion protein tail are suitable for use as potential drugs. By contrast, antibodies that trip the switch of the prion are very harmful and dangerous.
Literature:
Tiziana Sonati, Regina R. Reimann, Jeppe Falsig, Pravas Kumar Baral, Tracy O’Connor, Simone Hornemann, Sine Yaganoglu, Bei Li, Uli S. Herrmann, Barbara Wieland, Mridula Swayampakula, Muhammad Hafizur Rahman, Dipankar Das, Nat Kav, Roland Riek, Pawel P. Liberski, Michael N. G. James, and Adriano Aguzzi. The flexible tail of the prion protein mediates the toxicity of antiprion antibodies. Nature. July 31, 2013. Doi: 10.1038/nature12402
Contacts:
Prof. Adriano Aguzzi
Institute of Neuropathology
University of Zurich
Phone: +41 44 255 21 07
E-mail: adriano.aguzzi@usz.ch
Weitere Informationen:
http://www.mediadesk.uzh.ch/articles/2013/der-flexible-schweif-des-prions-vergiftet-hirnzellen_en.html
– News release of the University of Zurich in English, including video
http://www.mediadesk.uzh.ch/articles/2013/der-flexible-schweif-des-prions-vergiftet-hirnzellen.html
– News release of the University of Zurich in German, including video
Media Contact
More Information:
http://www.usz.chAll latest news from the category: Life Sciences and Chemistry
Articles and reports from the Life Sciences and chemistry area deal with applied and basic research into modern biology, chemistry and human medicine.
Valuable information can be found on a range of life sciences fields including bacteriology, biochemistry, bionics, bioinformatics, biophysics, biotechnology, genetics, geobotany, human biology, marine biology, microbiology, molecular biology, cellular biology, zoology, bioinorganic chemistry, microchemistry and environmental chemistry.
Newest articles

Why getting in touch with our ‘gerbil brain’ could help machines listen better
Macquarie University researchers have debunked a 75-year-old theory about how humans determine where sounds are coming from, and it could unlock the secret to creating a next generation of more…
Attosecond core-level spectroscopy reveals real-time molecular dynamics
Chemical reactions are complex mechanisms. Many different dynamical processes are involved, affecting both the electrons and the nucleus of the present atoms. Very often the strongly coupled electron and nuclear…
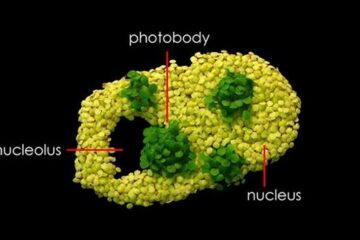
Free-forming organelles help plants adapt to climate change
Scientists uncover how plants “see” shades of light, temperature. Plants’ ability to sense light and temperature, and their ability to adapt to climate change, hinges on free-forming structures in their…