Can aging and metabolic processes be controlled? New insights into the activation of sirtuins
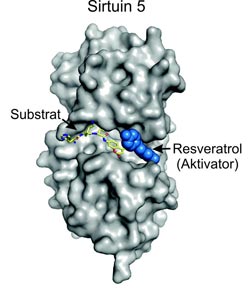
Graphics of a complex which alongside Sirt5 contains a peptide with fluorophore as a substrate and resveratrol as an activator.<br><br>Graphics: Department of Biochemistry, University of Bayreuth; free for publication when references are included.<br>
However, biochemical basic research is now one significant step closer to this distant goal. A research group led by Prof. Clemens Steegborn at the University of Bayreuth in Germany has now published a study in the online edition of the science magazine “PLOS ONE” that provides insight important for guidingfuture research in this field.
Sirtuins: Catalysts for cellular processes
One of the core functions of sirtuins, a special class of enzymes, is to control metabolic and aging processes. The human organism has seven different types of sirtuins, known in research as “Sirt1” to “Sirt7”. They change the structure of essential, survival proteins by splitting acetyl groups of these molecules at specific sites. This deacetylation process triggers important signals for cellular processes, such as for creating new proteins based on genetic information or adjusting the breakdown of nutrients. Medical and pharmaceutical researchers therefore have a growing interest in how sirtuins work and how they are affected by pharmacological drugs.
Many details of this connection between sirtuins and cellular processes, however, are still largely in the dark. Dr Melanie Gertz and Prof. Clemens Steegborn's team at the University of Bayreuth – in cooperation with scientists at Ruhr-University Bochum and Vienna University – have now made an important step towards shedding some light on this puzzle.
Amplifying and diminishing effects of resveratrol
At the heart of the research presented in “PLOS ONE” is resveratrol, a natural substance found in grapes and red wine, for instance. The scientists could show that resveratrol on the one hand can amplify the deacetyling effect of Sirt5 on peptides and proteins, but on the other hand suppresses the deacetyling effect of Sirt3. Their research has undermined a hypothesis based on previous research results, which suggested that resveratrol could only affect sirtuins in this way if the peptides were synthetically coupled with fluorescing molecules – known as fluorophores. The research that has now been published shows, however, that the effect of resveratrol does not depend on any such a modification.
The activity of the enzyme Sirt5 is artificially amplified
In a next step the researchers looked at this effect in more detail. They used protein crystallography to visualize the three dimensional structure of a complex which alongside Sirt5 contains a peptide with fluorophore as a substrate and resveratrol as an activator. In this complex, the substrate and the activator are directly next to each other. This triangle of interactions is extremely beneficial for the deacetyling effect of the sirtuin on the peptide.
To ensure that there is actually a deacetyling effect of the enzyme Sirt5, the peptide has to be accomodated into the enzyme's molecular structure – or to put it more accurately, into what is known as the peptide binding pocket. What is essential, however, is the appropriate spatial alignment of the peptide. Once the peptide has docked into the open binding pocket, resveratrol also binds there. Thereby, it closes this opening in the Sirt5 molecule and locks in the peptide. The resveratrol molecule also interacts directly with the peptide, and the resulting constellation amplifies the deacetyling effect of the sirtuin. It appears that resveratrol prevents the peptide from leaving the binding pocket before deacetylation by Sirt5; and it seems also to adjust the peptide in an orientation favourable for the deacetylation.
The interaction between resveratrol and the fluorophore-labelled peptide discovered by the researcher team also works if the substrate has not been artificially modified. This explains the observed activation of Sirt5 on natural peptides, in the form they occur in organisms.
A comparable mechanism attenuates the activity of the enzyme Sirt3
The biochemists in Bayreuth used the same methods to also analyse how resveratrol and related substances affect the deacetyling effect of Sirt3. In a three dimensional structure where Sirt3 takes the place of Sirt5, it is now piceatannol – a small molecule almost identical to resveratrol – that docks onto the sirtuin. The piceatannol interacts directly with the peptide, just as the resveratrol did in the previous structure. However, this time the deacetyling effect of the sirtuin is not amplified, but is actually suppressed.
So why do resveratrol / piceatannol have these opposing effects on Sirt5 and Sirt3? The researchers do not have a definitive answer, but they do have a first idea. The interactions resveratrol / piceatannol forms to the sirtuin on the one hand and to the peptide on the other hand lead to a slightly different peptide arrangement in Sirt3 when compared to Sirt5. The positioning of the substrate is crucial for the deacetylation; even a small change can therefore slow down Sirt3, but also speed up Sirt5.
The effect of resveratrol on the sirtuin depends on the substrate
The research team from Bayreuth has made another illuminating observation – the peptide, in its role as a substrate, interacts directly with the activator, suggesting that the type of peptide substrate would affect how the substances modify the deacetyling effect of the sirtuin. The scientists were indeed able to show such an influence of the substrate in initial experiments with Sirt5. Prof. Steegborn's group now plans to further test this mechanism and to analyze it in more detail.
A structural model for further research:
On the path towards targeted control
With the research results presented in “PLOS ONE”, there is for the first time a structural model that allows to understand the fundamental principles of the effects of resveratrol and similar substances on Sirt3 and Sirt5 – and also on other sirtuins, such as Sirt1, which has been the subject of extensive research – and to systematically continue these analyses. The authors consider this model an important basis for future studies. “We should now investigate various peptides and proteins to see how their deacetylation by sirtuins is stimulated or inhibited if resveratrol or similar substances act on these sirtuins”, explained Steegborn. He added: “The available results provoke an exciting idea for future research: The development of activators or inhibitors that are custom-made to specifically regulate the effects of Sirt3 and Sirt5 on individual, selected proteins. This approach would result in more specific effects than modifying the deacetylation of all substrate proteins in the same way and would thus help to prevent undesirable side effects. This would bring us an important step closer to the vision of precisely controling aging or metabolic processes with pharmacological drugs.”
Publication:
Melanie Gertz et al.,
A Molecular Mechanism for Direct Sirtuin Activation by Resveratrol,
in: PLOS ONE (2012), published online first on November 21, 2012
DOI: 10.1371/journal.pone.0049761
Contact for further information:
Prof. Dr. Clemens Steegborn
Department of Biochemistry
University of Bayreuth
D-95440 Bayreuth
Telephone: +49 (0) 921 / 55-2421
E-Mail: clemens.steegborn@uni-bayreuth.de
Media Contact
More Information:
http://www.uni-bayreuth.deAll latest news from the category: Life Sciences and Chemistry
Articles and reports from the Life Sciences and chemistry area deal with applied and basic research into modern biology, chemistry and human medicine.
Valuable information can be found on a range of life sciences fields including bacteriology, biochemistry, bionics, bioinformatics, biophysics, biotechnology, genetics, geobotany, human biology, marine biology, microbiology, molecular biology, cellular biology, zoology, bioinorganic chemistry, microchemistry and environmental chemistry.
Newest articles

Microscopic basis of a new form of quantum magnetism
Not all magnets are the same. When we think of magnetism, we often think of magnets that stick to a refrigerator’s door. For these types of magnets, the electronic interactions…

An epigenome editing toolkit to dissect the mechanisms of gene regulation
A study from the Hackett group at EMBL Rome led to the development of a powerful epigenetic editing technology, which unlocks the ability to precisely program chromatin modifications. Understanding how…

NASA selects UF mission to better track the Earth’s water and ice
NASA has selected a team of University of Florida aerospace engineers to pursue a groundbreaking $12 million mission aimed at improving the way we track changes in Earth’s structures, such…





















