Graphene-nanotube hybrid boosts lithium metal batteries
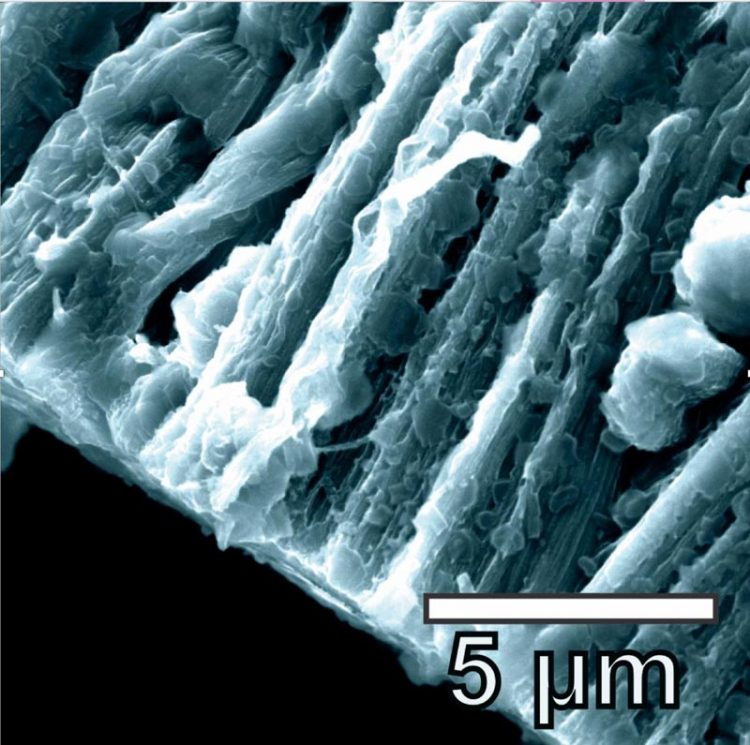
Lithium metal coats the hybrid graphene and carbon nanotube anode in a battery created at Rice University. The lithium metal coats the three-dimensional structure of the anode and avoids forming dendrites. Credit: Tour Group/Rice University
Rice University scientists have created a rechargeable lithium metal battery with three times the capacity of commercial lithium-ion batteries by resolving something that has long stumped researchers: the dendrite problem.
The Rice battery stores lithium in a unique anode, a seamless hybrid of graphene and carbon nanotubes. The material first created at Rice in 2012 is essentially a three-dimensional carbon surface that provides abundant area for lithium to inhabit.
The anode itself approaches the theoretical maximum for storage of lithium metal while resisting the formation of damaging dendrites or “mossy” deposits.
Dendrites have bedeviled attempts to replace lithium-ion with advanced lithium metal batteries that last longer and charge faster. Dendrites are lithium deposits that grow into the battery's electrolyte. If they bridge the anode and cathode and create a short circuit, the battery may fail, catch fire or even explode.
Rice researchers led by chemist James Tour found that when the new batteries are charged, lithium metal evenly coats the highly conductive carbon hybrid in which nanotubes are covalently bonded to the graphene surface.
As reported in the American Chemical Society journal ACS Nano, the hybrid replaces graphite anodes in common lithium-ion batteries that trade capacity for safety.
“Lithium-ion batteries have changed the world, no doubt,” Tour said, “but they're about as good as they're going to get. Your cellphone's battery won't last any longer until new technology comes along.”
He said the new anode's nanotube forest, with its low density and high surface area, has plenty of space for lithium particles to slip in and out as the battery charges and discharges. The lithium is evenly distributed, spreading out the current carried by ions in the electrolyte and suppressing the growth of dendrites.
Though the prototype battery's capacity is limited by the cathode, the anode material achieves a lithium storage capacity of 3,351 milliamp hours per gram, close to the theoretical maximum and 10 times that of lithium-ion batteries, Tour said. Because of the low density of the nanotube carpet, the ability of lithium to coat all the way down to the substrate ensures maximum use of the available volume, he said.
The researchers had their “Aha!” moment in 2014, when co-lead author Abdul-Rahman Raji, a former graduate student in Tour's lab and now a postdoctoral researcher at the University of Cambridge, began experimenting with lithium metal and the graphene-nanotube hybrid.
“I reasoned that lithium metal must have plated on the electrode while analyzing results of experiments carried out to store lithium ions in the anode material combined with a lithium cobalt oxide cathode in a full cell,” Raji said. “We were excited because the voltage profile of the full cell was very flat. At that moment, we knew we had found something special.”
Within a week, Raji and co-lead author Rodrigo Villegas Salvatierra, a Rice postdoctoral researcher, deposited lithium metal into a standalone hybrid anode so they could have a closer look with a microscope. “We were stunned to find no dendrites grown, and the rest is history,” Raji said.
To test the anode, the Rice lab built full batteries with sulfur-based cathodes that retained 80 percent capacity after more than 500 charge-discharge cycles, approximately two years' worth of use for a normal cellphone user, Tour said. Electron microscope images of the anodes after testing showed no sign of dendrites or the moss-like structures that have been observed on flat anodes. To the naked eye, anodes within the quarter-sized batteries were dark when empty of lithium metal and silver when full, the researchers reported.
“Many people doing battery research only make the anode, because to do the whole package is much harder,” Tour said. “We had to develop a commensurate cathode technology based upon sulfur to accommodate these ultrahigh-capacity lithium anodes in first-generation systems. We're producing these full batteries, cathode plus anode, on a pilot scale, and they're being tested.”
###
Co-authors of the paper are Rice postdoctoral researcher Nam Dong Kim, visiting researchers Xiujun Fan and Junwei Sha and graduate students Yilun Li and Gladys López-Silva. Tour is the T.T. and W.F. Chao Chair in Chemistry as well as a professor of computer science and of materials science and nanoengineering at Rice.
The Air Force Office of Scientific Research Multidisciplinary University Research Initiative supported the research.
Read the abstract at http://pubs.
This news release can be found online at http://news.
Follow Rice News and Media Relations via Twitter @RiceUNews
Related materials:
Tour Group: http://www.
Wiess School of Natural Sciences: http://natsci.
Images for download:
http://news.
A forest of carbon nanotubes grown on and covalently attached to a graphene substrate makes an excellent anode for high-capacity lithium metal batteries, according to Rice University researchers. The recyclable batteries show promise to safely provide far more capacity than present-day lithium ion batteries. (Credit: Tour Group/Rice University)
http://news.
Lithium metal coats the hybrid graphene and carbon nanotube anode in a battery created at Rice University. The lithium metal coats the three-dimensional structure of the anode and avoids forming dendrites. (Credit: Tour Group/Rice University)
http://news.
An electron microscope image shows a carbon nanotube evenly coated with lithium metal. Tests on the graphene-carbon nanotube anode created at Rice University show it resists the formation of lithium dendrites that can damage batteries. (Credit: Tour Group/Rice University)
http://news.
A graphic shows carbon nanotubes covalently bonded to a graphene substrate. The material created at Rice University is being tested as an anode for high-capacity lithium metal batteries. (Credit: Tour Group/Rice University)
Located on a 300-acre forested campus in Houston, Rice University is consistently ranked among the nation's top 20 universities by U.S. News & World Report. Rice has highly respected schools of Architecture, Business, Continuing Studies, Engineering, Humanities, Music, Natural Sciences and Social Sciences and is home to the Baker Institute for Public Policy. With 3,879 undergraduates and 2,861 graduate students, Rice's undergraduate student-to-faculty ratio is 6-to-1. Its residential college system builds close-knit communities and lifelong friendships, just one reason why Rice is ranked No. 1 for happiest students and for lots of race/class interaction by the Princeton Review. Rice is also rated as a best value among private universities by Kiplinger's Personal Finance. To read “What they're saying about Rice,” go to http://tinyurl.
Media Contact
All latest news from the category: Materials Sciences
Materials management deals with the research, development, manufacturing and processing of raw and industrial materials. Key aspects here are biological and medical issues, which play an increasingly important role in this field.
innovations-report offers in-depth articles related to the development and application of materials and the structure and properties of new materials.
Newest articles

A universal framework for spatial biology
SpatialData is a freely accessible tool to unify and integrate data from different omics technologies accounting for spatial information, which can provide holistic insights into health and disease. Biological processes…

How complex biological processes arise
A $20 million grant from the U.S. National Science Foundation (NSF) will support the establishment and operation of the National Synthesis Center for Emergence in the Molecular and Cellular Sciences (NCEMS) at…

Airborne single-photon lidar system achieves high-resolution 3D imaging
Compact, low-power system opens doors for photon-efficient drone and satellite-based environmental monitoring and mapping. Researchers have developed a compact and lightweight single-photon airborne lidar system that can acquire high-resolution 3D…





















