Angling chromium to let oxygen through
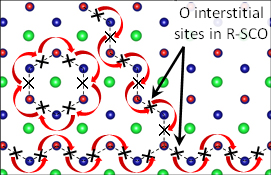
Routing: Oxygen can zigzag or take a circular route (red arrows) through this semiconducting crystal made of strontium (green), chromium (blue), and oxygen (red). Image Courtesy of Nature Communications
Researchers have been trying to increase the efficiency of solid oxide fuel cells by lowering the temperatures at which they run. More efficient fuel cells might gain wider use in vehicles or as quiet, pollution-free, neighborhood electricity generating stations. A serendipitous finding has resulted in a semiconducting material that could enable fuel cells to operate at temperatures two-thirds lower than current technology, scientists reported August 18 in Nature Communications.
In an attempt to create a metal oxide with the properties of metal, researchers at the Department of Energy's Pacific Northwest National Laboratory created a new form of the metal oxide. This particular strontium-chromium oxide performs as a semiconductor, or a material whose ability to conduct electricity can be turned on and off. It also allows oxygen to diffuse easily, a requirement for a solid oxide fuel cell. Best yet, it allows diffusion at a temperature that can lead to much more efficient fuel cells.
Nothing is Something
Energy researchers need improved materials to make fuel cells more widely used. Solid oxide fuel cells require oxides capable of absorbing and transmitting negatively charged oxygen atoms at low temperature. Current materials require temperatures around 800 degrees Celsius (for reference, car engines run at about 200 degrees Celsius and steel melts around 1500).
Researchers at PNNL were trying to make strontium chromium oxide in a kind of crystalline form called perovskite, which has many useful electronic properties. In this material, the strontium, chromium and oxygen atoms stack together in a cube. The metal atoms — strontium and chromium — bond completely to the oxygen atoms around them.
However, in the material that formed, the strontium chromium oxide packed into a rhombus-shaped crystal — think diamond — and many of the oxygen atoms were missing.
What's more, the holes where the oxygen atoms had been, also called oxygen vacancies, had come together to form well-defined planes within the new crystal structure. The researchers found that these planes act as channels that allow oxygen from outside the material to diffuse through the material at an exceptionally low temperature for these materials, about 250 degrees Celsius.
“At high enough concentrations, oxygen vacancies aggregate and form new mesoscale structures with novel properties that the original material doesn't have,” said PNNL materials scientist Scott Chambers, who led the research. “In this case, the mesoscale crystalline structure transmits oxygen very efficiently.”
Bad Angle Bonds
The scientists inadvertently generated the material by taking advantage of the natural tendency of chromium atoms to avoid certain bonding environments. They found that their attempts to make metallic SrCrO3 (strontium chromium oxide in a ratio of 1:1:3) lead instead to the formation of semiconducting SrCrO2.8 (with a ratio of 1:1:2.8).
Because chromium as an ion with a charge of +4 does not like to form 90o bonds with oxygen, as it must in SrCrO3, SrCrO2.8 forms instead with a completely different crystal structure. This material contains oxygen-deficient regions through which oxygen can diffuse very easily. Those regions might provide a way to take better advantage of the material's electronic properties.
“As an additional benefit, ordered arrays of oxygen vacancies might allow us to separate the material's electronic and thermal properties,” said Chambers. “This would help us improve the performance of thermoelectrics, in either generating power from heat or for use in refrigeration.”
The team made ultra-pure crystalline films of the new material and used instruments and expertise at EMSL, DOE's Environmental Molecular Sciences Laboratory, to understand the material's properties. A DOE Office of Science User Facility, EMSL scientists worked with Chambers to develop a new instrument called an oxygen-assisted molecular beam epitaxy deposition system that is specifically designed to make and study these kinds of crystalline films.
Towards Light and Electrons
In the future, the team plans to apply the understanding gained to other materials, such as the deposition, characterization, and understanding of epitaxial strontium-doped lanthanum chromite, which has potential importance in visible light harvesting.
In the long term, the team plans to exploit the observed phenomenon to carry out nanofabrication of novel heterogeneous catalytic structures by depositing submonolayer quantities of catalytically important metals on the surface of rhombus-shaped, semiconducting oxide, and using the intersection of the defect planes with the free surface to order the incoming metal atoms into nanowires.
This work was supported by the Department of Energy Office of Science, EMSL and PNNL.
Reference: Hong-Liang Zhang, Peter V. Sushko, RJ Colby, Yingge Du, Mark E. Bowden , and Scott A. Chambers. 2014. Reversible Nano-Structuring of SrCrO3-δ Through Oxidization and Reduction at Low Temperatures. Nature Communications, August 18, 2014. DOI: 10.1038/ncomms5669.
Media Contact
More Information:
http://www.pnl.gov/news/release.aspx?id=1072All latest news from the category: Materials Sciences
Materials management deals with the research, development, manufacturing and processing of raw and industrial materials. Key aspects here are biological and medical issues, which play an increasingly important role in this field.
innovations-report offers in-depth articles related to the development and application of materials and the structure and properties of new materials.
Newest articles

A universal framework for spatial biology
SpatialData is a freely accessible tool to unify and integrate data from different omics technologies accounting for spatial information, which can provide holistic insights into health and disease. Biological processes…

How complex biological processes arise
A $20 million grant from the U.S. National Science Foundation (NSF) will support the establishment and operation of the National Synthesis Center for Emergence in the Molecular and Cellular Sciences (NCEMS) at…

Airborne single-photon lidar system achieves high-resolution 3D imaging
Compact, low-power system opens doors for photon-efficient drone and satellite-based environmental monitoring and mapping. Researchers have developed a compact and lightweight single-photon airborne lidar system that can acquire high-resolution 3D…





















