New Take on Impacts of Low Dose Radiation
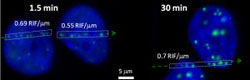
Imaging of a cell’s DNA damage response to radiation shows that 1.5 minutes after irradiation, the sizes and intensities of radiation induced foci (RIF) are small and weak, but 30 minutes later damage sites have clustered into larger and brighter RIF, probably reflecting DNA repair centers. <br>
Researchers with the U.S. Department of Energy (DOE)’s Lawrence Berkeley National Laboratory (Berkeley Lab), through a combination of time-lapse live imaging and mathematical modeling of a special line of human breast cells, have found evidence to suggest that for low dose levels of ionizing radiation, cancer risks may not be directly proportional to dose. This contradicts the standard model for predicting biological damage from ionizing radiation – the linear-no-threshold hypothesis or LNT – which holds that risk is directly proportional to dose at all levels of irradiation.
“Our data show that at lower doses of ionizing radiation, DNA repair mechanisms work much better than at higher doses,” says Mina Bissell, a world-renowned breast cancer researcher with Berkeley Lab’s Life Sciences Division. “This non-linear DNA damage response casts doubt on the general assumption that any amount of ionizing radiation is harmful and additive.”
Bissell was part of a study led by Sylvain Costes, a biophysicist also with Berkeley Lab’s Life Sciences Division, in which DNA damage response to low dose radiation was characterized simultaneously across both time and dose levels. This was done by measuring the number of RIF, for “radiation induced foci,” which are aggregations of proteins that repair double strand breaks, meaning the DNA double helix is completely severed.
“We hypothesize that contrary to what has long been thought, double strand breaks are not static entities but will rapidly cluster into preferred regions of the nucleus we call DNA repair centers as radiation exposure increases,” says Costes. “As a result of this clustering, a single RIF may reflect a center where multiple double strand breaks are rejoined. Such multiple repair activity increases the risks of broken DNA strands being incorrectly rejoined and that can lead to cancer.”
Costes and Bissell have published the results of their study in the Proceedings of the National Academy of Sciences in a paper titled “Evidence for formation of DNA repair centers and dose-response nonlinearity in human cells.” Also co-authoring the paper were Teresa Neumaier, Joel Swenson, Christopher Pham, Aris Polyzos, Alvin Lo, PoAn Yang, Jane Dyball, Aroumougame Asaithamby, David Chen and Stefan Thalhammer.
The authors believe their study to be the first to report the clustering of DNA double strand breaks and the formation of DNA repair centers in human cells. The movement of the double strand breaks across relatively large distances of up to two microns led to more intensely active but fewer RIF. For example, 15 RIF per gray (Gy) were observed after exposure to two Gy of radiation, compared to approximately 64 RIF/Gy after exposure to 0.1Gy. One Gy equals one joule of ionizing radiation energy absorbed per kilogram of human tissue. A typical mammogram exposes a patient to about 0.01Gy.
Corresponding author Costes says the DNA repair centers may be a logical product of evolution.
“Humans evolved in an environment with very low levels of ionizing radiation, which makes it unlikely that a cell would suffer more than one double strand break at any given time,” he says. “A DNA repair center would seem to be an optimal way to deal with such sparse damage. It is like taking a broken car to a garage where all the equipment for repairs is available rather than to a random location with limited resources.”
However, when cells are exposed to ionizing radiation doses large enough to cause multiple double strand breaks at once, DNA repair centers become overwhelmed and the number of incorrect rejoinings of double strand breaks increases.
“It is the same as when dozens of broken cars are brought to the same garage at once, the quality of repair is likely to suffer,” Costes says.
Mina Bissell is Berkeley Lab’s “Distinguished Scientist” and one of the world’s foremost breast cancer researchers. (Photo by Roy Kaltschmidt, Berkeley Lab)
The link between exposure to ionizing radiation and DNA damage that can give rise to cancerous cells is well-established. However, the standards for cancer risks have been based on data collected from survivors of the atomic bomb blasts in Japan during World War II. The LNT model was developed to extrapolate low dose cancer risk from high dose exposure because changes in cancer incidence following low dose irradiation are too small to be measurable. Extrapolation was done on a linear scale in accordance with certain assumptions and the laws of physics.
“Assuming that the human genome is a target of constant size, physics predicts DNA damage response will be proportional to dose leading to a linear scale,” Costes explains. “Epidemiological data from the survivors of the atomic bombs was found to be in agreement with this hypothesis and showed that cancer incidence increases with an increase in ionizing radiation dose above 0.1 Gy. Below such dose, the picture is not clear.”
Previous studies failed to detect the clustering of double break strands and the formation of DNA repair centers because they were based on single-time or single-dose measurements of RIF at a discrete time after the initial exposure to ionizing radiation. This yields a net number of RIF that does not account for RIF that have not yet appeared or RIF that have already made repairs and disappeared. The time-lapse imaging used by Costes, Bissell and their co-authors showed that RIF formation continues to occur well beyond the initial radiation exposure and after earlier repair issues have been resolved. Time-lapse imaging also indicates that double strand break clustering takes place before any RIF are formed.
“We hypothesize that double strand break clustering occurs rapidly after exposure to ionizing radiation and that RIF formation reflects the repair machinery put in place around a single cluster of double strand breaks,” Costes says. “Our results provide a more accurate model of RIF dose response, and underscore fundamental concerns about static image data analysis in the dynamic environment of the living cell.”
Previous studies also mostly involved fibroblast cells whereas Costes, Bissell and their colleagues examined epithelial cells, specifically an immortalized human breast cell line known as MCF10A, which has a much higher background of RIF than fibroblasts, even without ionizing irradiation. To compensate for this higher background, Costes developed a mathematical method that enables background to be corrected for on a per- nucleus basis in unirradiated cells. Still the use of a special line of immortalized breast cells is an issue that Costes and his colleagues plan to address.
“We are now looking at primary breast epithelial cells that have been removed from healthy donors to determine if our results are repeated beyond just a single cell line and under more realistic physiological conditions,” Costes says. “We’d also like to know if our findings hold true for fibroblasts as well as epithelial cells. Also, we’d like to know if double strand break clustering is the result of a random coalescence or if there is an active transport mechanism that moves these double strand breaks towards pre-existing DNA repair centers.”
Working in collaboration with Rafael Gomez-Sjoberg of Berkeley Lab’s Engineering Division, Costes and his group are also developing a special microfluidics lab-on-a-chip device that is integrated into an X-ray microbeam. The goal is to provide a means by which cells can be kept in a controlled microenvironment while being irradiated with multiple doses. This microfluidic array will be used to characterize DNA damage response in breast and blood cells collected from human donors.
“By characterizing DNA damage response in cells from many different human donors,” Costes says, “we should be able to determine the variation across humans and gain a better understanding of how sensitivity to DNA damage from ionizing radiation might vary from individual to individual.”
This research was supported by the DOE Office of Science.
Lawrence Berkeley National Laboratory addresses the world’s most urgent scientific challenges by advancing sustainable energy, protecting human health, creating new materials, and revealing the origin and fate of the universe. Founded in 1931, Berkeley Lab’s scientific expertise has been recognized with 13 Nobel prizes. The University of California manages Berkeley Lab for the U.S. Department of Energy’s Office of Science. For more, visit www.lbl.gov.
DOE’s Office of Science is the single largest supporter of basic research in the physical sciences in the Unites States, and is working to address some of the most pressing challenges of our time. For more information, please visit the Office of Science website at science.energy.gov.
Additional Information:
For more information about the research of Sylvain Costes visit the Website at http://biocomp.lbl.gov
For more information about the research of Mina Bissell visit the Website at http://www.lbl.gov/LBL-Programs/lifesciences/BissellLab/main.html
For more about the DOE Office of Science Low Dose Radiation Research Program visit the Website at http://lowdose.energy.gov/
Media Contact
More Information:
http://www.lbl.govAll latest news from the category: Health and Medicine
This subject area encompasses research and studies in the field of human medicine.
Among the wide-ranging list of topics covered here are anesthesiology, anatomy, surgery, human genetics, hygiene and environmental medicine, internal medicine, neurology, pharmacology, physiology, urology and dental medicine.
Newest articles

Trotting robots reveal emergence of animal gait transitions
A four-legged robot trained with machine learning by EPFL researchers has learned to avoid falls by spontaneously switching between walking, trotting, and pronking – a milestone for roboticists as well…

Innovation promises to prevent power pole-top fires
Engineers in Australia have found a new way to make power-pole insulators resistant to fire and electrical sparking, promising to prevent dangerous pole-top fires and reduce blackouts. Pole-top fires pose…

Possible alternative to antibiotics produced by bacteria
Antibacterial substance from staphylococci discovered with new mechanism of action against natural competitors. Many bacteria produce substances to gain an advantage over competitors in their highly competitive natural environment. Researchers…





















