Ultraviolet protection molecule in plants yields its secrets to Scripps research team
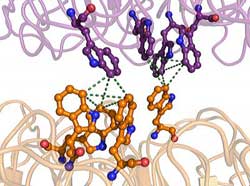
Scientists from Scripps Research and the University of Glasgow have put together a detailed picture of a UV-sensing protein known as UVR8, helping to explain mysteries such as how plant growth varies with sunlight. Credit: Image courtesy of the Getzoff lab, the Scripps Research Institute.<br>
Ultraviolet (UV) radiation from the sun can damage proteins and DNA inside cells, leading to poor growth and even death (as well as carcinogenesis in humans). But plants have evolved some powerful adaptive defenses, including a complex array of protective responses orchestrated by a UV-sensing protein molecule known as UVR8. Now, scientists from The Scripps Research Institute and the University of Glasgow have put together a detailed picture of UVR8's structure and inner workings.
“It's an ancient molecule that seems to play a fundamental role in plants,” said Scripps Research Professor Elizabeth Getzoff. “Knowing how it works helps us to understand better how plant growth varies with changes in sunlight, for example due to climate shifts; it's also important that we understand its basic light-switch mechanism.”
Getzoff was a principal investigator for the study, which is reported February 9 in the journal Science's early online edition, Science Express.
Sunscreen for Plants
Researchers first found evidence of UVR8's protective function in 2002, when they knocked out its gene in the wild mustard plant Arabidopsis, the standard experimental model for plant biologists. The mutant plants grew poorly when exposed to UV “B” wavelength radiation—the range most responsible for tanning and burning of human skin. When UVR8 is present in Arabidopsis, it can sense UV-B light and switch on a broad protective response involving more than 100 Arabidopsis genes. “These are genes for DNA-repair enzymes and other protective proteins,” said Getzoff. “It's the plant equivalent of putting on sunscreen.”
Molecules similar to UVR8 have been found in more ancient plant species such as algae and mosses, suggesting that UVR8 represents a primordial adaptation to UV light, possibly originating before Earth's atmosphere developed a UV-absorbing ozone layer.
John Christie of the University of Glasgow was a visiting scientist in Getzoff's lab in 2010, and suggested a collaboration to find out more about UVR8. Getzoff's lab specializes in finding and analyzing the detailed atomic structures of proteins, while Christie and his colleague Gareth I. Jenkins, a professor of plant cell and molecular biology at the University of Glasgow, are experts on UVR8 biology.
Structures No One Has Seen Before
In the study, Christie and other members of Getzoff's lab produced and purified copies of the UVR8 molecule and chemically induced it to crystallize—to line up in a regular pattern. Firing X-rays at the crystallized UVR8, evaluating the resulting diffraction pattern, and using related techniques, the scientists were able to determine UVR8's molecular architecture, including the three-dimensional arrangement of its component atoms, to a fine resolution of 1.7 Angstroms—170 trillionths of a meter.
UVR8 was known to be a “dimer” made of two identical protein subunits. The Getzoff lab's structural analysis revealed that these subunits are doughnut-shaped, and normally stick together weakly, like a couple of balloons that have become electrically charged after being rubbed. The interface connecting these two subunits is spanned by the component amino acids of the protein—including pyramidal structures made of tryptophan amino acids, which serve as the primary sensors of UV-B light. “The absorption of UV-B photons by these tryptophan pyramids leads to the weakening of the electrostatic force that holds the two UVR8 subunits together,” said Christie.
As a result of this weakening, the subunits can separate, move singly to the cell nucleus, and begin their orchestration of gene activity. The team's analysis also suggested that within a few hours, the subunits can reassemble into UV-B-sensitive dimers again.
“Other light-sensing proteins require a chemical modification or helper molecule to detect light, but UVR8 is unique in that it has these inbuilt UV-B-sensing tryptophan pyramids—structures that no one has seen before,” said Christie.
Molecule with Its Own Light Sensor
To help confirm that UVR8 can sense UV-B light entirely on its own, members of Jenkins's lab altered single amino acids within UVR8 to see how the molecule's light-sensing function changed. Tryptophans in the pyramid structure turned out to be crucial for UV-B detection; in fact, amino-acid substitution of one tryptophan by a phenylalanine shifts the sensitivity of UVR8 to shorter-wavelength UV-C radiation. “These experiments showed without a doubt that UVR8 contains its own light sensor,” said Getzoff.
Getzoff and her colleagues now intend to find out more precisely how the absorption of UV-B causes the disassociation of the UVR8 dimer, and then how the separated subunits interact with other proteins and chromosomes in the nucleus to switch on protective responses in the plant. Both UVR8 and a similar, but not UV-controlled, protein in humans bind to chromosomes to control gene activity.
In addition to Getzoff, Christie, and Jenkins, the other co-authors of the paper, “Plant UVR8 Photoreceptor Senses UV-B by Tryptophan-mediated Disruption of Cross-Dimer Salt Bridges,” were Andrew S. Arvai, Ashley J. Pratt, and Kenichi Hitomi, of the Getzoff lab at Scripps Research; Katherine J. Baxter, Monica Heilmann, and Andrew O'Hara of the Jenkins lab at the University of Glasgow; Brian Smith and Sharon M. Kelly of the Institute of Molecular, Cell and Systems Biology at the University of Glasgow; and Michael Hothorn of the Salk Institute for Biological Studies in La Jolla, California.
The study was supported by funds from the National Institutes of Health and the National Science Foundation; the Skaggs Institute for Chemical Biology at Scripps Research; the Royal Society and the Leverhulme Trust.
About The Scripps Research Institute
The Scripps Research Institute is one of the world's largest independent, non-profit biomedical research organizations. Scripps Research is internationally recognized for its discoveries in immunology, molecular and cellular biology, chemistry, neuroscience, and vaccine development, as well as for its insights into autoimmune, cardiovascular, and infectious disease. Headquartered in La Jolla, California, the institute also includes a campus in Jupiter, Florida, where scientists focus on drug discovery and technology development in addition to basic biomedical science. Scripps Research currently employs about 3,000 scientists, staff, postdoctoral fellows, and graduate students on its two campuses. The institute's graduate program, which awards Ph.D. degrees in biology and chemistry, is ranked among the top ten such programs in the nation. For more information, see http://www.scripps.edu
Media Contact
More Information:
http://www.scripps.eduAll latest news from the category: Life Sciences and Chemistry
Articles and reports from the Life Sciences and chemistry area deal with applied and basic research into modern biology, chemistry and human medicine.
Valuable information can be found on a range of life sciences fields including bacteriology, biochemistry, bionics, bioinformatics, biophysics, biotechnology, genetics, geobotany, human biology, marine biology, microbiology, molecular biology, cellular biology, zoology, bioinorganic chemistry, microchemistry and environmental chemistry.
Newest articles

Microscopic basis of a new form of quantum magnetism
Not all magnets are the same. When we think of magnetism, we often think of magnets that stick to a refrigerator’s door. For these types of magnets, the electronic interactions…

An epigenome editing toolkit to dissect the mechanisms of gene regulation
A study from the Hackett group at EMBL Rome led to the development of a powerful epigenetic editing technology, which unlocks the ability to precisely program chromatin modifications. Understanding how…

NASA selects UF mission to better track the Earth’s water and ice
NASA has selected a team of University of Florida aerospace engineers to pursue a groundbreaking $12 million mission aimed at improving the way we track changes in Earth’s structures, such…





















