The coldest reaction
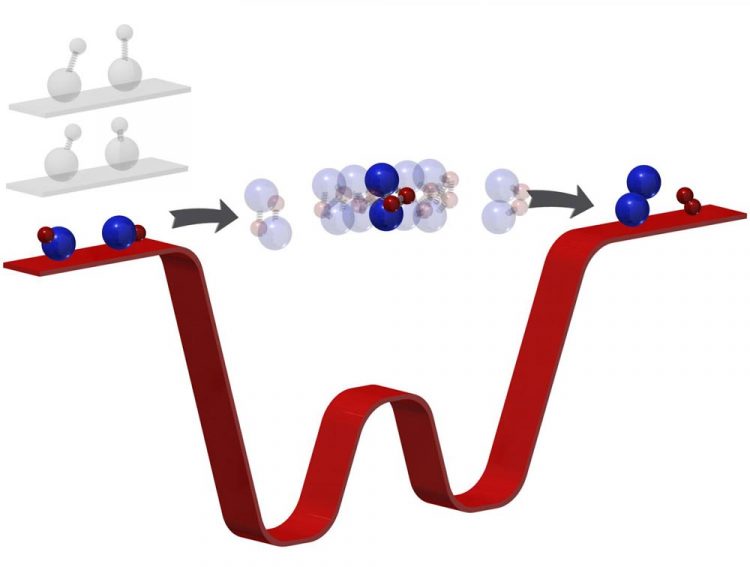
Chemical reactions transform reactants to products through an intermediate state where bonds break and form. Often too short-lived to observe, this phase has so-far eluded intimate investigation. By "freezing out" the rotation, vibration, and motion of the reactants (here, potassium-rubidium molecules) to a temperature of 500 nanokelvin (barely above absolute zero temperature), the number of energetically allowed exits for the products is limited. "Trapped" in the intermediate for far longer, researchers can then observe this phase directly with photoionization detection. This technique paves the way towards the quantum control of chemical reactions with ultracold molecules. Credit: Ming-Guang Hu
The coldest chemical reaction in the known universe took place in what appears to be a chaotic mess of lasers. The appearance deceives: Deep within that painstakingly organized chaos, in temperatures millions of times colder than interstellar space, Kang-Kuen Ni achieved a feat of precision. Forcing two ultracold molecules to meet and react, she broke and formed the coldest bonds in the history of molecular couplings.
“Probably in the next couple of years, we are the only lab that can do this,” said Ming-Guang Hu, a postdoctoral scholar in the Ni lab and first author on their paper published today in Science.
Five years ago, Ni, the Morris Kahn Associate Professor of Chemistry and Chemical Biology and a pioneer of ultracold chemistry, set out to build a new apparatus that could achieve the lowest temperature chemical reactions of any currently available technology. But they couldn't be sure their intricate engineering would work.
Now, they not only performed the coldest reaction yet, they discovered their new apparatus can do something even they did not predict. In such intense cold–500 nanokelvin or just a few millionths of a degree above absolute zero–their molecules slowed to such glacial speeds, Ni and her team could see something no one has been able to see before: the moment when two molecules meet to form two new molecules. In essence, they captured a chemical reaction in its most critical and elusive act.
Chemical reactions are responsible for literally everything: breathing, cooking, digesting, creating energy, pharmaceuticals, and household products like soap. So, understanding how they work at a fundamental level could help researchers design combinations the world has never seen.
With an almost infinite number of new combinations possible, these new molecules could have endless applications from more efficient energy production to new materials like mold-proof walls and even better building blocks for quantum computers.
In her previous work, Ni used colder and colder temperatures to work this chemical magic: forging molecules from atoms that would otherwise never react. Cooled to such extremes, atoms and molecules slow to a quantum crawl, their lowest possible energy state.
There, Ni can manipulate molecular interactions with utmost precision. But even she could only see the start of her reactions: two molecules go in, but then what? What happened in the middle and the end was a black hole only theories could try to explain.
Chemical reactions occur in just millionths of a billionth of a second, better known in the scientific world as femtoseconds. Even today's most sophisticated technology can't capture something so short-lived, though some come close.
In the last twenty years, scientists have used ultra-fast lasers like fast-action cameras, snapping rapid images of reactions as they occur. But they can't capture the whole picture. “Most of the time,” Ni said, “you just see that the reactants disappear and the products appear in a time that you can measure. There was no direct measurement of what actually happened in these chemical reactions.” Until now.
Ni's ultracold temperatures force reactions to a comparatively numbed speed. “Because [the molecules] are so cold,” Ni said, “now we kind of have a bottleneck effect.” When she and her team reacted two potassium rubidium molecules–chosen for their pliability–the ultracold temperatures forced the molecules to linger in the intermediate stage for microseconds.
Microseconds–mere millionths of a second–may seem short, but that's millions of times longer than usual and long enough for Ni and her team to investigate the phase when bonds break and form, in essence, how one molecule turns into another.
With this intimate vision, Ni said she and her team can test theories that predict what happens in a reaction's black hole to confirm if they got it right. Then, her team can craft new theories, using actual data to more precisely predict what happens during other chemical reactions, even those that take place in the mysterious quantum realm.
Already, the team is exploring what else they can learn in their ultracold test bed. Next, for example, they could manipulate the reactants, exciting them before they react to see how their heightened energy impacts the outcome. Or, they could even influence the reaction as it occurs, nudging one molecule or the other.
“With our controllability, this time window is long enough, we can probe,” Hu said. “Now, with this apparatus, we can think about this. Without this technique, without this paper, we cannot even think about this.”
Media Contact
All latest news from the category: Life Sciences and Chemistry
Articles and reports from the Life Sciences and chemistry area deal with applied and basic research into modern biology, chemistry and human medicine.
Valuable information can be found on a range of life sciences fields including bacteriology, biochemistry, bionics, bioinformatics, biophysics, biotechnology, genetics, geobotany, human biology, marine biology, microbiology, molecular biology, cellular biology, zoology, bioinorganic chemistry, microchemistry and environmental chemistry.
Newest articles

Looking inside battery cells
The power of combining different views. Lithium-Ion batteries presently are the ubiquitous source of electrical energy in mobile devices, and the key technology for e-mobility and energy storage. Massive interdisciplinary…

New snail-inspired robot can climb walls
A robot, designed to mimic the motion of a snail, has been developed by researchers at the University of Bristol. Adding to the increasing innovative new ways robots can navigate,…

New technique improves finishing time for 3D printed machine parts
North Carolina State University researchers have demonstrated a technique that allows people who manufacture metal machine parts with 3D printing technologies to conduct automated quality control of manufactured parts during…





















