Nerve cells' gatekeepers take many forms
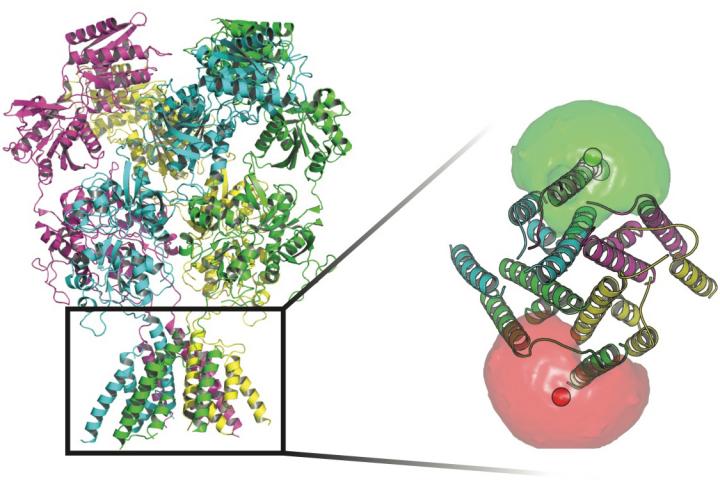
Scientists at Rice University and UTHealth tracked the conformations of proteins that stand guard at transmembrane channels in the walls of nerve cells. At the base of the proteins in this image are fluorophores (red and green spheres) surrounded by fluorophore clouds that helped the researchers define the movements of the gate that allows ions to flow through the membrane. Credit: Rice University/UTHealth
The ability to track the movements of single molecules has revealed how proteins on the surface of nerve cells control gates that turn chemical signals into electrical signals. The finding is a step forward in detailing mechanisms involved in neurological disease, according to researchers at Rice University and the University of Texas Health Science Center at Houston (UTHealth).
Using sophisticated imaging and statistical methods, the scientists employed single-molecule FRET imaging techniques to establish a beachhead at the NMDA receptor gate that, when activated, allows ions to flow through the nerve cell's membrane.
FRET stands for Förster resonance energy transfer. It is a way to use the light emitted by two fluorescent-tagged molecules as a sensitive ruler for very small distances, such as the opening in the NMDA receptor channel.
Rice chemist Christy Landes, an expert in single-molecule FRET, and Vasanthi Jayaraman, a professor of biochemistry and molecular biology at UTHealth's McGovern Medical School, whose expertise is in NMDA receptor biochemistry, teamed to gather the first experimental evidence detailing the dynamics of how the receptors alter their shapes to control the sensitivity of the gate to chemical signals. The new study appears in Nature Chemical Biology.
The NMDA receptor is set of four protein subunits, each with four domains, and each of those domains has a particular function. Collectively, they span the cell membrane. Each subunit can have many “states,” or shapes, that regulate which electrical signals — and how many of them — pass through. The subunits sit on each side of the channel and activate when they bind both glutamate and glycine neurotransmitter ligands and trigger the signaling pathway that allows positively charged ions to pass into the cell.
“These receptors are critical for normal physiological function,” Jayaraman said. “A lot of times you may not want to turn signaling on or off. You may want to dial in the extent of signaling. Once we understand all the protein's states, we can start thinking about ways to do this, thus keeping the protein active but to varying degrees as needed.
“It's important for drug development to understand these dynamics because the motions and the energetic properties of these proteins dictate their specific functions,” she said. “We were able to do both.”
This knowledge could lead to multifunctional drugs that influence the channels in subtle ways, Landes said. Known NMDA receptor antagonists include common anesthetics, synthetic opioids like methadone and dissociative drugs like ketamine and nitrous oxide. Depressed NMDA receptor function is suspected in memory deficits commonly associated with aging. Alcohol is known to inhibit glutamate, one of two neurotransmitters that bind to NMDA.
“A lot of drug design has as its core principle that there's one way to bind, and you basically either turn something on or turn something off,” Landes said. “But it's obvious that this type of receptor protein isn't just on or off. There are multiple conformational interactions that either improve or degrade the signaling.”
In an earlier study, the team analyzed the conformations of a smaller and simpler but related system, the C-clamp-like agonist binding domain of another receptor, AMPA. AMPA mediates fast signal transmission in the central nervous system. The single-molecule FRET technique allowed the researchers to get the first snapshots of the AMPA protein's various clamp conformations at rest and also when bound to a range of target molecules by measuring the distance between two light-activated molecular tags.
This time, the researchers sought to understand the channel opening itself — how the proteins that make up the NMDA receptor channel move to activate the ion gate. By adding fluorescent tags across the channel and sampling them over time, they were able to map the energy landscape of the transmembrane segment of the protein in its resting state or under the influence of ligands that modulate the gate among open, closed and intermediate states. Each channel's structural state directly influences the electrical signals that are allowed to pass.
They discovered that the agonist-free (resting) state is structurally stiff, which confirmed its energetic resistance to adopting conformations that would allow channel opening. Agonists like glutamate are the target chemicals that trigger electrical signal transport through the channel. The researchers confirmed that in the presence of the primary agonist, the NMDA channel was less stiff and therefore able to transition more easily among the possible channel-open conformations.
The work also showed how two modulators known to interact with different parts of the full receptor impact channel conformation and stiffness. Zinc ions, despite binding to the extracellular portion of the receptor far away from the channel, induce a stiff channel with significant energetic barriers to opening, similar to the receptor's resting state. In contrast, the “pore blocker” dizocilpine was found to enhance the energetics of transitions among multiple conformations that don't relate to channel opening.
Landes said that rather than capture static states, as is typically done with X-rays, “We were looking at distance changes across the channel in a dynamic fashion. It's much more complicated.” That gave them a dataset more akin to a movie than a snapshot, she said.
She said the study required new techniques to purify and stabilize the full proteins, which were drawn from the neurons of rodents. “There were three key pieces to this project: handling the full proteins, getting them purified and labeled on the single-receptor level and performing the data analysis to find out what it means.
“That's truly the foundation here,” she said. “Now we can do these measurements for much more complicated systems.”
###
Drew Dolino, an alumnus of the University of Texas MD Anderson Cancer Center UTHealth Graduate School of Biomedical Sciences, and graduate student Sudeshna Chatterjee of Rice are lead authors of the paper. Co-authors are postdoctoral fellows David MacLean and Sana Shaikh of the Center for Membrane Biology, Department of Biochemistry and Molecular Biology, at the McGovern Medical School at UTHealth, and graduate students Charlotte Flatebo and Logan Bishop of Rice. Landes, a professor of chemistry and of electrical and computer engineering at Rice, and Jayaraman, a professor of biochemistry and molecular biology at the Center for Membrane Biology, McGovern Medical School, are co-senior authors.
The National Institutes of Health, the American Heart Association, the Schissler Foundation and the Welch Foundation supported the research.
Read the abstract at http://dx.
DOI: 10.1038/nchembio.2487
This news release can be found online at http://news.
Follow Rice News and Media Relations via Twitter @RiceUNews
Related materials:
Researchers get a grip on nervous system's receptors: http://news.
Landes Research Group: http://www.
Jayaraman Lab: https:/
Wiess School of Natural Sciences: http://naturalsciences.
McGovern Medical School: https:/
Images for download:
http://news.
Christy Landes, left, and Vasanthi Jayaraman. (Credit: Jeff Fitlow/Rice University)
http://news.
Scientists at Rice University and UTHealth tracked the conformations of proteins that stand guard at transmembrane channels in the walls of nerve cells. At the base of the proteins in this image are fluorophores (red and green spheres) surrounded by fluorophore clouds that helped the researchers define the movements of the gate that allows ions to flow through the membrane. (Credit: Rice University/UTHealth)
http://news.
he four subunits of NMDA receptor proteins can each have many states that regulate which electrical signals pass through the membrane of a nerve cell. The subunits activate when they bind both glutamate and glycine neurotransmitter ligands and trigger the signaling pathway that allows positively charged ions to pass into the cell. (Credit: Rice University/UTHealth)
Established in 1972 by The University of Texas System Board of Regents, The University of Texas Health Science Center at Houston (UTHealth) is Houston's Health University and Texas' resource for health care education, innovation, scientific discovery and excellence in patient care. The most comprehensive academic health center in the UT System and the U.S. Gulf Coast region, UTHealth is home to schools of biomedical informatics, biomedical sciences, dentistry, nursing and public health and the John P. and Kathrine G. McGovern Medical School. UTHealth includes The University of Texas Harris County Psychiatric Center, as well as the growing clinical practices UT Physicians, UT Dentists and UT Health Services. The university's primary teaching hospitals are Memorial Hermann-Texas Medical Center, Children's Memorial Hermann Hospital and Harris Health Lyndon B. Johnson Hospital. For more information, visit http://www.
Located on a 300-acre forested campus in Houston, Rice University is consistently ranked among the nation's top 20 universities by U.S. News & World Report. Rice has highly respected schools of Architecture, Business, Continuing Studies, Engineering, Humanities, Music, Natural Sciences and Social Sciences and is home to the Baker Institute for Public Policy. With 3,879 undergraduates and 2,861 graduate students, Rice's undergraduate student-to-faculty ratio is 6-to-1. Its residential college system builds close-knit communities and lifelong friendships, just one reason why Rice is ranked No. 1 for quality of life and for lots of race/class interaction and No. 2 for happiest students by the Princeton Review. Rice is also rated as a best value among private universities by Kiplinger's Personal Finance. To read “What they're saying about Rice,” go to http://tinyurl.
Media Contact
All latest news from the category: Life Sciences and Chemistry
Articles and reports from the Life Sciences and chemistry area deal with applied and basic research into modern biology, chemistry and human medicine.
Valuable information can be found on a range of life sciences fields including bacteriology, biochemistry, bionics, bioinformatics, biophysics, biotechnology, genetics, geobotany, human biology, marine biology, microbiology, molecular biology, cellular biology, zoology, bioinorganic chemistry, microchemistry and environmental chemistry.
Newest articles

Microscopic basis of a new form of quantum magnetism
Not all magnets are the same. When we think of magnetism, we often think of magnets that stick to a refrigerator’s door. For these types of magnets, the electronic interactions…

An epigenome editing toolkit to dissect the mechanisms of gene regulation
A study from the Hackett group at EMBL Rome led to the development of a powerful epigenetic editing technology, which unlocks the ability to precisely program chromatin modifications. Understanding how…

NASA selects UF mission to better track the Earth’s water and ice
NASA has selected a team of University of Florida aerospace engineers to pursue a groundbreaking $12 million mission aimed at improving the way we track changes in Earth’s structures, such…





















