Drug discovery: Alzheimer's and Parkinson's spurred by same enzyme
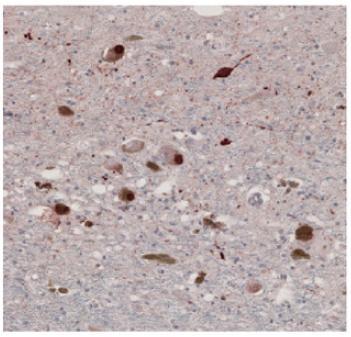
This is a Parkinson's disease brain sample, stained with an antibody that only recognizes the N103 chunk of alpha-synuclein, which is generated through cleavage by AEP. Credit: From Zhang et al NSMB (2017).
Alzheimer's disease and Parkinson's disease are not the same. They affect different regions of the brain and have distinct genetic and environmental risk factors.
But at the biochemical level, these two neurodegenerative diseases start to look similar. That's how Emory scientists led by Keqiang Ye, PhD, landed on a potential drug target for Parkinson's.
In both Alzheimer's (AD) and Parkinson's (PD), a sticky protein forms toxic clumps in brain cells. In AD, the troublemaker inside cells is called tau, making up neurofibrillary tangles. In PD, the sticky protein is alpha-synuclein, forming Lewy bodies.
Ye and his colleagues had previously identified an enzyme (asparagine endopeptidase or AEP) that trims tau in a way that makes it more sticky and toxic. Drugs that inhibit AEP have beneficial effects in Alzheimer's animal models.
In a new Nature Structural and Molecular Biology paper, Emory researchers show that AEP acts in the same way toward alpha-synuclein.
“In Parkinson's, alpha-synuclein behaves much like Tau in Alzheimer's,” Ye says. “We reasoned that if AEP cuts Tau, it's very likely that it will cut alpha-synuclein too.”
A particular chunk of alpha-synuclein produced by AEP's scissors can be found in samples of brain tissue from patients with PD, but not in control samples, Ye's team found.
In control brain samples AEP was confined to lysosomes, parts of the cell with a garbage disposal function. But in PD samples, AEP was leaking out of the lysosomes to the rest of the cell.
The researchers also observed that the chunk of alpha-synuclein generated by AEP is more likely to aggregate into clumps than the full length protein, and is more toxic when introduced into cells or mouse brains. In addition, alpha-synuclein mutated so that AEP can't cut it is less toxic.
Ye cautions that AEP is not the only enzyme that cuts alpha-synuclein into various toxic pieces, and the full-length alpha-synuclein protein is still able to aggregate and cause harm. Nevertheless, he says his team is moving on to testing drugs that inhibit AEP in Parkinson's animal models.
###
First author Zhentao Zhang, MD, PhD, a former postdoc with Ye, is now at Wuhan University in China.
At Emory, the laboratories of P. Michael Iuvone, PhD in the Department of Ophthalmology and Nick Seyfried, PhD in the Department of Biochemistry contributed to the paper. Lingjing Jin, MD at Shanghai Tongji Hospital and Jian-Zhi Wang, MD, Key Laboratory of Ministry of Education of China for Neurological Disorders also contributed to the paper.
The research was supported by the Michael J. Fox Foundation, the National Natural Science Foundation of China and the National Eye Institute (P30EY006360 and R01EY004864).
Media Contact
All latest news from the category: Life Sciences and Chemistry
Articles and reports from the Life Sciences and chemistry area deal with applied and basic research into modern biology, chemistry and human medicine.
Valuable information can be found on a range of life sciences fields including bacteriology, biochemistry, bionics, bioinformatics, biophysics, biotechnology, genetics, geobotany, human biology, marine biology, microbiology, molecular biology, cellular biology, zoology, bioinorganic chemistry, microchemistry and environmental chemistry.
Newest articles

Sea slugs inspire highly stretchable biomedical sensor
USC Viterbi School of Engineering researcher Hangbo Zhao presents findings on highly stretchable and customizable microneedles for application in fields including neuroscience, tissue engineering, and wearable bioelectronics. The revolution in…

Twisting and binding matter waves with photons in a cavity
Precisely measuring the energy states of individual atoms has been a historical challenge for physicists due to atomic recoil. When an atom interacts with a photon, the atom “recoils” in…
Nanotubes, nanoparticles, and antibodies detect tiny amounts of fentanyl
New sensor is six orders of magnitude more sensitive than the next best thing. A research team at Pitt led by Alexander Star, a chemistry professor in the Kenneth P. Dietrich…





















