Researchers Watch Catalysts at Work
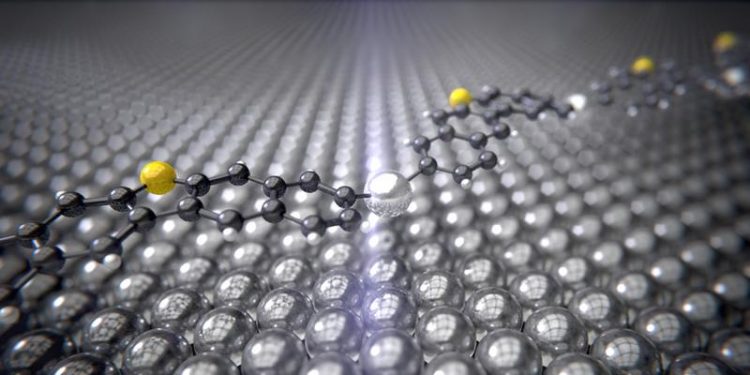
The intermediate product of the Ullmann reaction with the silver catalyst (silver) between the carbon rings (black) and sulfur atoms (yellow) curves like a bridge over the silver surface. University of Basel, Department of Physics
The Ullmann reaction examined is a chemical reaction in which silver atoms catalyze the bond between two carbon atoms to which iodine was previously bonded. Although scientists have known about this type of reaction since 1901 and used it for many important chemical conversions, it was not previously possible to observe the intermediate product of the reaction in detail.
Using an atomic force microscope, the team of researchers led by Professor Ernst Meyer and Dr. Shigeki Kawai from the Swiss Nanoscience Institute and the Department of Physics at the University of Basel has now succeeded in displaying this reaction at atomic resolution.
Surprisingly, it was revealed that the silver atoms react with the molecules at temperatures of around -120°C and seem to curve like a bridge over a river. In the second stage of the reaction, which requires the temperature to be increased to around 105°C and generates the end product, the silver atoms are freed again and two carbon atoms bond together.
Calculating energy
The Ullmann reaction has been used for chemical syntheses for a long time now. Interest in this linking of carbon atoms has recently increased again because it can be used to bind organic molecules to surfaces and produce solvent-free polymers. Detailed observations of how the catalysts work enable scientists to better understand the reaction process.
Previous analyses failed to show the spatial arrangement of the organometallic intermediate product. The detailed images now obtained are the first to allow project partner Professor Stefan Goedecker (Department of Physics, University of Basel) to calculate the energy turnover of the Ullmann reaction examined. This data confirms the unusual spatial arrangement of the intermediate product and indicates how the reaction could be optimized.
Relatively low temperatures
The observed curving and flexibility of the molecules is probably the reason why the reaction requires relatively low temperatures of 105°C. The molecules are subject to mechanical tension and can therefore react more easily, that is at lower temperatures. If other catalysts could be used to generate intermediate products like these that are subject to tension, then catalytic reactions could also be possible at lower temperatures.
This would make ecological and economic sense because traditional catalysts with platinum, rhodium, or palladium often require high operating temperatures of 500°C, which leads to the emission of waste gases in a cold state.
The research work was the result of a collaboration between the Department of Physics at the University of Basel, the National Institute of Materials Science (Japan), the Japan Science and Technology Agency (Japan), the University of Tokyo (Japan), and Shadid Beheshti University (Iran).
Original source
Shigeki Kawai, Ali Sadeghi, Toshihiro Okamoto, Chikahiko Mitsui, Rémy Pawlak, Tobias Meier, Jun Takeya, Stefan Goedecker and Ernst Meyer
Organometallic Bonding in an Ullmann-Type On-Surface Chemical Reaction Studied by High-Resolution Atomic Force Microscopy
Small (2016), DOI: 10.1002/smll.201601216
Further information
Professor Ernst Meyer, University of Basel, Department of Physics, tel. +41 61 267 37 24, email: ernst.meyer@unibas.ch
https://www.unibas.ch/en/News-Events/News/Uni-Research/Basel-Researchers-Watch-C…
Media Contact
All latest news from the category: Physics and Astronomy
This area deals with the fundamental laws and building blocks of nature and how they interact, the properties and the behavior of matter, and research into space and time and their structures.
innovations-report provides in-depth reports and articles on subjects such as astrophysics, laser technologies, nuclear, quantum, particle and solid-state physics, nanotechnologies, planetary research and findings (Mars, Venus) and developments related to the Hubble Telescope.
Newest articles

Why getting in touch with our ‘gerbil brain’ could help machines listen better
Macquarie University researchers have debunked a 75-year-old theory about how humans determine where sounds are coming from, and it could unlock the secret to creating a next generation of more…
Attosecond core-level spectroscopy reveals real-time molecular dynamics
Chemical reactions are complex mechanisms. Many different dynamical processes are involved, affecting both the electrons and the nucleus of the present atoms. Very often the strongly coupled electron and nuclear…
Free-forming organelles help plants adapt to climate change
Scientists uncover how plants “see” shades of light, temperature. Plants’ ability to sense light and temperature, and their ability to adapt to climate change, hinges on free-forming structures in their…





















