Directly Visualizing Hydrogen Bonds
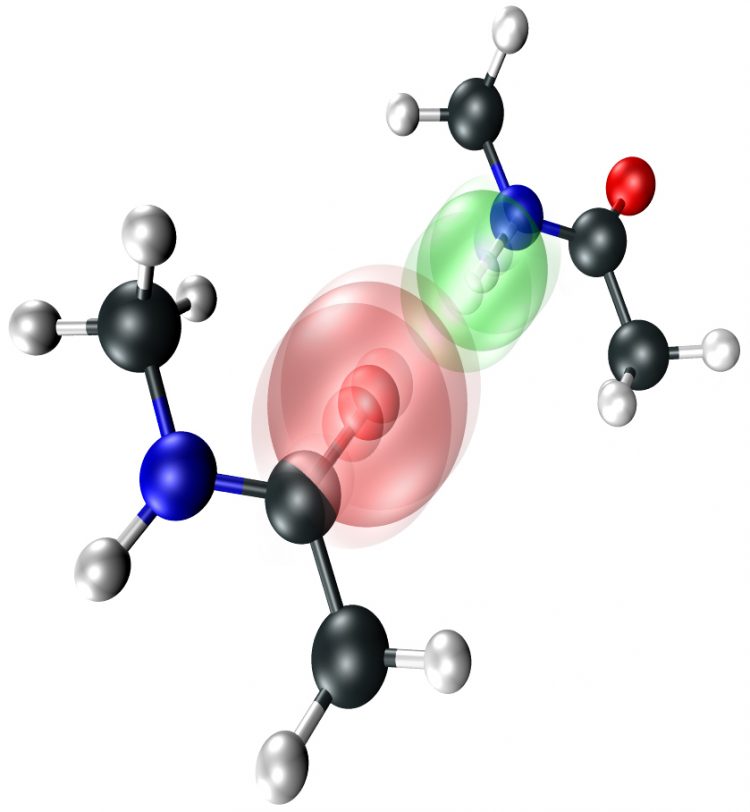
L. De Marco/UChicago The hydrogen-bonding interaction causes the atoms on each individual N-methylacetamide molecule to vibrate in unison.
Using a newly developed, ultrafast femtosecond infrared light source, chemists at the University of Chicago have been able to directly visualize the coordinated vibrations between hydrogen-bonded molecules — the first time this sort of chemical interaction, which is found in nature everywhere at the molecular level, has been directly visualized. They describe their experimental techniques and observations in The Journal of Chemical Physics, from AIP Publishing.
“These two-dimensional infrared spectroscopy techniques provide a new avenue to directly visualize both hydrogen bond partners,” said Andrei Tokmakoff, the lab’s primary investigator. “They have the spectral content and bandwidth to really interrogate huge parts of the vibrational spectrum of molecules. It’s opened up the ability to look at how very different types of vibrations on different molecules interact with one another.”
Tokmakoff and his colleagues sought to use two-dimensional infrared spectroscopy to directly characterize structural parameters such as intermolecular distances and hydrogen-bonding configurations, as this information can be encoded in intermolecular cross-peaks that spectroscopy detects between solute-solvent vibrations.
“You pluck on the bonds of one molecule and watch how it influences the other,” Tokmakoff said. “In our experiment, you’re basically plucking on both because they’re so strongly bound.”
Hydrogen bonds are typically perceived as the attractive force between the slightly negative and slightly positive ends of neutrally-charged molecules, such as water. While water stands apart with its unique polar properties, hydrogen bonds can form between a wide range of molecules containing electronegative atoms and range from weakly polar to nearly covalent in strength. Hydrogen bonding plays a key role in the action of large, biologically-relevant molecules and is often an important element in the discovery of new pharmaceuticals.
For their initial visualizations, Tokmakoff’s group used N-methylacetamide, a molecule called a peptide that forms medium-strength hydrogen-bonded dimers in organic solution due to its polar nitrogen-hydrogen and carbon-oxygen tails. By using a targeted three-pulse sequence of mid-infrared light and apparatus described in their article, Tokmakoff’s group was able to render the vibrational patterns of the two peptide units.
“All of the internal vibrations of hydrogen bonded molecules that we look at become intertwined, inextricably; you can’t think of them as just a simple sum of two parts,” Tokmakoff said.
Future work in Tokmakoff’s group involves visualizing the dynamics and structure of water around biological molecules such as proteins and DNA.
“You can’t just think of the water as sort of an amorphous solvent, you really have to at least on some level think of it atomistically and treat it that way,” Tokmakoff said. “And if you believe that, it has huge consequences all over the place, particularly in biology, where so much computational biology ignores the fact that water has real structure and real quantum mechanical properties of its own.”
The article, “Direct observation of intermolecular interactions mediated by hydrogen bonding,” is authored by Luigi De Marco, Martin Thämer, Mike Reppert and Andrei Tokmakoff. It will be published in The Journal of Chemical Physics on July 15, 2014. After that date, it can be accessed at: http://scitation.aip.org/content/aip/journal/jcp/141/3/10.1063/1.4885145
Authors of this study are affiliated with the Massachusetts Institute of Technology and the University of Chicago.
ABOUT THE JOURNAL
The Journal of Chemical Physics publishes concise and definitive reports of significant research in the methods and applications of chemical physics. See: http://jcp.aip.org
Media Contact
All latest news from the category: Physics and Astronomy
This area deals with the fundamental laws and building blocks of nature and how they interact, the properties and the behavior of matter, and research into space and time and their structures.
innovations-report provides in-depth reports and articles on subjects such as astrophysics, laser technologies, nuclear, quantum, particle and solid-state physics, nanotechnologies, planetary research and findings (Mars, Venus) and developments related to the Hubble Telescope.
Newest articles

Microscopic basis of a new form of quantum magnetism
Not all magnets are the same. When we think of magnetism, we often think of magnets that stick to a refrigerator’s door. For these types of magnets, the electronic interactions…

An epigenome editing toolkit to dissect the mechanisms of gene regulation
A study from the Hackett group at EMBL Rome led to the development of a powerful epigenetic editing technology, which unlocks the ability to precisely program chromatin modifications. Understanding how…

NASA selects UF mission to better track the Earth’s water and ice
NASA has selected a team of University of Florida aerospace engineers to pursue a groundbreaking $12 million mission aimed at improving the way we track changes in Earth’s structures, such…





















