Yeast model offers clues to possible drug targets for Lou Gehrig's disease, study shows

Now researchers at the Stanford University School of Medicine and San Francisco's Gladstone Institutes have used baker's yeast — a tiny, one-celled organism — to identify a chink in the armor of the currently incurable disease that may eventually lead to new therapies for human patients.
“Even though yeast and humans are separated by a billion years of evolution, we were able to use the power of yeast genetics to identify an unexpected potential drug target for ALS,” said Aaron Gitler, PhD, an associate professor of genetics at Stanford. “Many neurodegenerative disorders such as ALS, Parkinson's and Alzheimer's exhibit protein clumping or misfolding within the neurons that is thought to either cause or contribute to the conditions. We are trying to figure out why these proteins aggregate in neurons in the brain and spinal cord, and what happens when they do.”
In 2008, Gitler received a New Innovator award from the National Institutes of Health to use yeast as a model for understanding human neurodegenerative diseases and as a way to identify new targets for drug development.
Gitler is the co-senior author of the research, which will be published online Oct. 28 in Nature Genetics. Robert Farese, Jr., MD, a senior investigator at the Gladstone Institutes, is the other co-senior author. Stanford graduate student Maria Armakola shares co-first authorship with Matthew Higgins, PhD, a postdoctoral scholar at Gladstone.
Most cases of ALS have no clear-cut cause. However, it has recently been shown that an RNA-binding protein called TDP-43 accumulates in clumps in the cytoplasm of spinal cord neurons in many people with the condition, and mutations in this protein have been found in some people with the ALS. Researchers like Gitler and Farese have been able to mimic the disease in yeast by expressing TDP-43 at higher-than-normal levels, which causes the protein to form lethal clumps in the cells' cytoplasm.
“In humans, the progression of the disease can take years before symptoms arise,” said Gitler. “But in yeast, we see protein clumping in the cytoplasm within two days and the cells rapidly begin to die.” With their model system in place, Gitler and Farese set out to see whether it was possible to protect yeast cells from this effect by tinkering with the function of other proteins in the cell.
In this study, the researchers discovered that blocking the production of a protein called Dbr1 in a yeast model stops the TDP-43 clumping and allows the cells to live normally. The researchers confirmed the results in human nerve cells grown in the laboratory and in rat neurons overexpressing TDP-43.
“In this study we made no assumptions as to how TDP-43 injures cells,” said Farese, “but instead screened the whole yeast genome to find genes that might prevent the toxicity. Independently, both our lab and the Gitler lab found that loss of Dbr1, an enzyme involved in RNA processing, could do this.”
Dbr1 serves as part of the cellular clean-up crew that mops up the bits of unwanted RNA generated as part of the protein production line. In our DNA, most genes consist of coding regions, called exons, broken up into several segments by non-coding regions, called introns. Cells can make many different, related proteins from the same stretch of DNA by mixing and matching different exons in a process called splicing.
When the DNA is first copied, or transcribed, into RNA, the introns as well as the exons are included. But the cell quickly splices out the introns, which are released into the cytoplasm as little loops, or lariats. Dbr1, in turn, clips the loops to open them and make them accessible to the cell's disposal system.
Blocking the production of Dbr1 causes the RNA lariats to build up in the cytoplasm. The researchers showed — by creating lariats with a binding site for a fluorescent tracking protein — that the mutant TDP-43 binds to these excess lariats rather than clumping. The effect is like using a paper towel to mop up a spill on your computer keyboard: binding to the lariats appears to keep TDP-43 from causing havoc elsewhere.
“Normally, TDP-43 is found in the nucleus,” said first author Armakola. “But in the diseased cells, it aggregates in the cytoplasm and forms clumps. We developed a novel way to track where these lariats go in living cells, and we saw that when Dbr1 is missing, the lariats act as a sink to sequester TDP-43.”
The researchers note that it's still not entirely clear whether the cells die because the mutant TDP-43 is drawing essential RNA transcripts or regulatory molecules away from the nucleus and into the cytoplasm, or because it's not performing its normal RNA-binding function in the nucleus. Both could contribute to the progression of the disease.
The results in the yeast, rodent and human cells, however, suggest that therapeutic approaches aimed at blocking Dbr1 function, or even creating artificial lariat-like formations to draw away the mutant molecule, should be explored.
“Next, we'd like to explore blocking Dbr1 function in animals such as flies, worms and rodents,” said Armakola. “We're also interested in identifying small molecule inhibitors of Dbr1.”
Other Stanford co-authors include graduate student Matthew Figley. The research was supported by the NIH, the Ellison Medical Foundation, the Packard Center for ALS Research at Johns Hopkins, the Consortium for Frontotemporal Research, the ALS Association, the Taube-Koret Center, the Hellman Family Foundation, the Pew Charitable Trusts, the Rita Allen Foundation, the Searle Scholars Program, the Keck Foundation and the National Center for Research Resources.
The Stanford University School of Medicine consistently ranks among the nation's top medical schools, integrating research, medical education, patient care and community service. For more news about the school, please visit http://mednews.stanford.edu. The medical school is part of Stanford Medicine, which includes Stanford Hospital & Clinics and Lucile Packard Children's Hospital. For information about all three, please visit http://stanfordmedicine.org/about/news.html
Media Contact
More Information:
http://www.stanford.eduAll latest news from the category: Health and Medicine
This subject area encompasses research and studies in the field of human medicine.
Among the wide-ranging list of topics covered here are anesthesiology, anatomy, surgery, human genetics, hygiene and environmental medicine, internal medicine, neurology, pharmacology, physiology, urology and dental medicine.
Newest articles

Why getting in touch with our ‘gerbil brain’ could help machines listen better
Macquarie University researchers have debunked a 75-year-old theory about how humans determine where sounds are coming from, and it could unlock the secret to creating a next generation of more…
Attosecond core-level spectroscopy reveals real-time molecular dynamics
Chemical reactions are complex mechanisms. Many different dynamical processes are involved, affecting both the electrons and the nucleus of the present atoms. Very often the strongly coupled electron and nuclear…
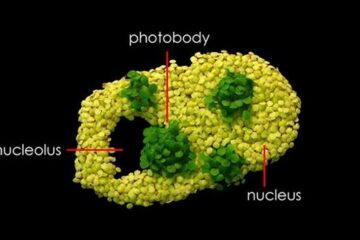
Free-forming organelles help plants adapt to climate change
Scientists uncover how plants “see” shades of light, temperature. Plants’ ability to sense light and temperature, and their ability to adapt to climate change, hinges on free-forming structures in their…