Purdue scientists: To stop cancer, keep your Icmt away from your Ras
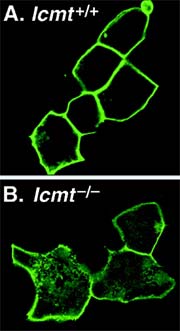
These two images show the development of human cells with (above) and without (below) the normal concentration of the protein Ras in their cell membranes. The cells on the right have the protein Ras scattered throughout their cytoplasm, in contrast with the normally developed cells on the left. The absence of the enzyme Icmt prevents Ras from lodging in the membrane, where it ordinarily performs tasks vital to cellular reproduction. Purdue research suggests that preventing Icmt from interacting with Ras during the protein’s development may inhibit the runaway cell growth that is characteristic of cancer, thus leading to new approaches to treating several forms of the disease. (M. Bergo, Journal of Biological Chemistry) <br>
Halting the development of certain pancreatic, ovarian, colon and lung cancers may be possible with therapy based on recent Purdue University research.
By investigating a single molecule that influences cell growth, a research group in the Purdue Cancer Center, including Brian S. Henriksen, has gained new insight into the chain of events that make some cancer cells divide uncontrollably – insight that may eventually lead to a way to break that chain, stopping cancer in its tracks. The molecule, known as Icmt, has a critical role in the development of Ras, an ordinarily beneficial protein that tells a cell to divide. The research group has determined how to inhibit Icmt’s influence on Ras, without which the protein cannot develop effectively into an instigator of cell growth.
“A tumor can be seen as cells that forget to stop dividing, and misdeveloped Ras is responsible for some instances of uncontrolled growth,” said Henriksen, a graduate student in medicinal chemistry and medical pharmacology in Purdue’s School of Pharmacy. “When Ras develops a mutation, it does its job incorrectly, and it becomes a hazard to the body. Our work with Icmt might lead to therapies that could stop errant Ras from causing tumors to progress.”
The research was conducted by an interdisciplinary team from two Purdue departments. Co-directing the team are Christine A. Hrycyna, Walther Assistant Professor in the School of Science’s chemistry department and Richard A. Gibbs, associate professor in the School of Pharmacy’s medicinal chemistry and molecular pharmacology department. Jessica L. Anderson (chemistry) and Henriksen are the principal graduate students involved in the project.
Henriksen will present the group’s results at 6 p.m. Sunday (3/28) at the 227th national meeting of the American Chemical Society in Anaheim, Calif.
Ras is a key protein that signals the body’s cells to begin or cease dividing, but for Ras to develop into its complex final form, it undergoes a lengthy chain of modifications that must play out correctly if the protein is ultimately to function properly.
Ras has long been known to be associated with cancer, as it is the incorrectly modified, or mutated, Ras that is behind the uncontrolled cell growth within many cancers. Ninety percent of all pancreatic cancers, one-half of all colon cancers and one-half of the most virulent lung cancers can be traced back to mutant Ras.
“If you want to fight cancer, controlling Ras is an attractive approach,” Gibbs said. “Since it requires a chain of modifications, researchers have long taken the approach that if you break the chain, you can stop the cancer.”
Earlier attempts to break the chain have met with difficulty because at the point scientists tried to break it in the past, there was always a “back way” – another chemical “route” the body’s enzymes could take to accomplish a given step in the process. Scientists tried to stop mutant Ras from maturing one way, only to find that they had been circumvented by another enzyme that also could get the job done.
“These troubles were actually a tribute to cellular life’s tenacity and survival techniques,” Henriksen said. “Life, cancerous life included, will often find a way to overcome adversity. That is why we are so excited about Icmt – it seems to be a weak link in the chain.”
The enzyme Icmt, short for isoprenyl cysteine methyltransferase, represents a link in the chain that is a bottleneck of sorts, as there appears to be no back way available to form Ras if Icmt is not present at the right point in the process. Specifically, Icmt’s task – a process called methyl esterification – is to add a small chemical “cap,” called a methyl group, to the larger Ras molecule. Without this methyl cap, Ras is unable to anchor itself in the plasma membrane that surrounds a cell, where it must lodge if it is to effect growth commands. So Gibbs and Hrycyna’s labs joined forces to block the biochemical activity of Icmt with small drug molecules, preventing the methyl esterification of Ras and effectively breaking its ability to localize to the plasma membrane.
“This effort depended on both labs,” Anderson said. “The Gibbs laboratory brought synthetic expertise, and the Hrycyna laboratory brought large quantities of the protein and the expertise to assay the effects of the inhibitors.”
Other groups have shown recently that this lack of a methyl cap causes Ras to localize in the wrong region of the cell and, importantly, results in a Ras protein that cannot support cancerous growth.
“These findings are terrific news for our efforts,” said Hrycyna. “If our inhibitor molecules directed at stopping or slowing the activity of Icmt in tumor cells are effective, we should be able to stop the growth of Ras-based cancers.”
Hrycyna said she believed the discovery could ultimately throw a wrench into cancer’s operation, but that it was still too early to predict victory.
“We have succeeded in stopping Ras at the protein level and are just beginning to work with whole cells,” she said. “This is very different from getting results in animals, and we’re still a long way from human trials. No one should look for this to cure cancer anytime soon, though we are extremely encouraged by these initial results.”
But to move the process toward the goal of curing cancer, the group plans to look into other ways of altering Icmt to increase the effect.
“Other ways of changing Icmt might make it even more potent at stopping the development of Ras,” Henriksen said. “We’d like to look at all our options and combine them into the most potent inhibitor we can manage – that’s a good goal for the near future.”
This research is sponsored in part by a collaborative grant to Gibbs and Hrycyna by the Indiana Elks and the Purdue Cancer Center.
The research group is associated with the Purdue Cancer Center. One of just eight National Cancer Institute-designated basic research facilities in the United States, the center attempts to help cancer patients by identifying new molecular targets and designing future agents and drugs for effectively detecting and treating cancer.
Writer: Chad Boutin, (765) 494-2081, cboutin@purdue.edu
Sources: Brian S. Henriksen, (765) 496-2727, loxarr@hotmail.com
Christine Hrycyna, (765) 494-7322, hrycyna@purdue.edu
Richard Gibbs, (765) 494-1456, rgibbs@purdue.edu
Jessica Anderson, jeanders@purdue.edu
Purdue News Service: (765) 494-2096; purduenews@purdue.edu
Media Contact
More Information:
http://news.uns.purdue.edu/html4ever/2004/040328.Henriksen.ras.htmlAll latest news from the category: Health and Medicine
This subject area encompasses research and studies in the field of human medicine.
Among the wide-ranging list of topics covered here are anesthesiology, anatomy, surgery, human genetics, hygiene and environmental medicine, internal medicine, neurology, pharmacology, physiology, urology and dental medicine.
Newest articles

Why getting in touch with our ‘gerbil brain’ could help machines listen better
Macquarie University researchers have debunked a 75-year-old theory about how humans determine where sounds are coming from, and it could unlock the secret to creating a next generation of more…

Attosecond core-level spectroscopy reveals real-time molecular dynamics
Chemical reactions are complex mechanisms. Many different dynamical processes are involved, affecting both the electrons and the nucleus of the present atoms. Very often the strongly coupled electron and nuclear…

Free-forming organelles help plants adapt to climate change
Scientists uncover how plants “see” shades of light, temperature. Plants’ ability to sense light and temperature, and their ability to adapt to climate change, hinges on free-forming structures in their…





















