Viewing a catalytic reaction in action
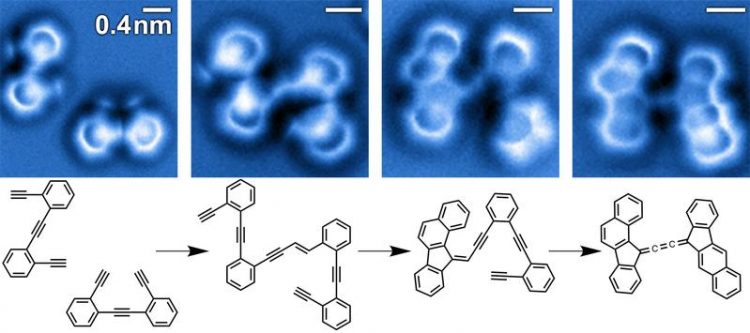
Identification of the different transient intermediates in a stepwise bimolecular enediyne coupling and cyclization cascade on a silver surface by non-contact atomic force microscopy. Image: A. Riss/TU Munich, adapted from A. Riss et al., Nature Chemistry (2016), DOI: 10.1038/nchem.2506
To be able to follow and directly visualize how the structure of molecules changes when they undergo complex chemical transformations has been a long-standing goal of chemistry. While reaction intermediates are particularly difficult to identify and characterize due to their short lifetimes, knowledge of the structure of these species can give valuable insights into reaction mechanisms and therefore impact fields beyond the chemical industry, such as materials science, nanotechnology, biology and medicine.
Now an international team of researchers led by Felix R. Fischer, Michael F. Crommie (University of California at Berkeley and Lawrence Berkeley National Laboratory), and Angel Rubio (Director at the Max Planck Institute for the Structure and Dynamics of Matter at CFEL in Hamburg and Distinguished Professor at the University of the Basque Country in San Sebastián) has imaged and resolved the bond configuration of reactants, intermediates and final products of a complex and technologically relevant organic surface reaction at the single-molecule level. The findings are published in the journal Nature Chemistry today.
Chemical transformations at the interface between solid/liquid or solid/gaseous phases of matter lie at the heart of key industrial-scale manufacturing processes. The microscopic mechanism of this surface-catalyzed organic reaction is a grand challenge for modern heterogeneous catalysis and its application to industrial-scale chemical processes.
Competing pathways that lead to numerous intermediates and undesired side products often hamper investigation of the underlying reaction mechanisms that transform crude feedstock into complex value-added chemicals at the surface of a heterogeneous catalyst bed. The precise structural identification of transient reaction intermediates and products, however, is limited by their respective concentrations in the sample stream.
In the present work, the chemical structures associated with different steps of a multistep reaction cascade of enediyne molecules on a silver surface were imaged using non-contact atomic force microscopy (nc-AFM) with special functionalized tips (using a carbon monoxide molecule to enhance resolution).
Identification of the precise bond configuration of the intermediate species has allowed the determination of the intricate sequence of chemical transformations along the pathway from reactants via intermediates to end products and thus allowed unraveling the microscopic mechanisms behind that intricate dynamical behavior.
“It was striking to be able to directly measure and theoretically characterize the chemical structure of reaction intermediates in this complex system,” said Felix Fischer, Professor for Chemistry at the University of Berkeley California and co-lead author of the study.
“This is a huge step for chemical synthesis,” added co-lead author Angel Rubio, Director at the Max Planck Institute for the Structure and Dynamics of Matter in Hamburg and Distinguished Professor for Physics at the University of the Basque Country. “However, we wanted to go deeper and understand why the intermediates are stabilized on the surface – this does not happen in solution.”
A combination of extensive state-of-the-art numerical calculations with classic analytical models describing the kinetics of sequential chemical reactions has shown that it is not enough to consider the energy potential landscape (i.e. the energies of the species along the reaction pathway and the associated transformation barriers), but that energy dissipation to the substrate and changes in molecular entropy play a critical role for the stabilization of the intermediates.
The surface, and in particular the interaction of molecular radicals with the surface, plays a key role for both, entropy and selective dissipation, highlighting fundamental differences of surface-supported reactions compared to gas-phase or solution chemistry.
Such detailed understanding constitutes a fundamental milestone in the analysis of chemical reactions that was achieved through the synergy between single-molecule visualization of chemical reactions and state-of-the-art high-performance computer modeling.
By these means, many limitations of conventional ensemble averaging spectroscopic techniques are surpassed, and an unprecedented atomic-scale picture of the reaction mechanisms, driving forces and kinetics emerges. Such new insight may open countless of hitherto unexplored venues for the future design and optimization of heterogeneous catalytic systems, for the development of novel synthetic tools applied to carbon-based nanotechnology, as well as for biochemical and materials science applications.
Contact person:
Prof. Angel Rubio
Max Planck Institute for the Structure and Dynamics of Matter
Center for Free-Electron Laser Science
Luruper Chaussee 149
22761 Hamburg
Germany
+49 (0)40 8998-6550
angel.rubio@mpsd.mpg.de
Original publication:
A. Riss, A. Pérez Paz, S. Wickenburg, H.-Z. Tsai, D. G. de Oteyza, A. J. Bradley, M. M. Ugeda, P. Gorman, H. S. Jung, M. F. Crommie, A. Rubio, and F. R. Fischer, “Imaging single-molecule reaction intermediates stabilized by surface dissipation and entropy,” Nature Chemistry, Advance Online Publication (May 2, 2016), DOI: 10.1038/nchem.2506
http://dx.doi.org/10.1038/nchem.2506 Original publication
http://www.mpsd.mpg.de/en/research/theo Research group of Prof. Angel Rubio
http://www.mpsd.mpg.de/en Max Planck Institute for the Structure and Dynamics of Matter
Media Contact
All latest news from the category: Life Sciences and Chemistry
Articles and reports from the Life Sciences and chemistry area deal with applied and basic research into modern biology, chemistry and human medicine.
Valuable information can be found on a range of life sciences fields including bacteriology, biochemistry, bionics, bioinformatics, biophysics, biotechnology, genetics, geobotany, human biology, marine biology, microbiology, molecular biology, cellular biology, zoology, bioinorganic chemistry, microchemistry and environmental chemistry.
Newest articles

Microscopic basis of a new form of quantum magnetism
Not all magnets are the same. When we think of magnetism, we often think of magnets that stick to a refrigerator’s door. For these types of magnets, the electronic interactions…

An epigenome editing toolkit to dissect the mechanisms of gene regulation
A study from the Hackett group at EMBL Rome led to the development of a powerful epigenetic editing technology, which unlocks the ability to precisely program chromatin modifications. Understanding how…

NASA selects UF mission to better track the Earth’s water and ice
NASA has selected a team of University of Florida aerospace engineers to pursue a groundbreaking $12 million mission aimed at improving the way we track changes in Earth’s structures, such…





















