Treatments for HIV-visceral leishmaniasis co-infected patients

Migrant workers are one of the highly vulnerable, HIV-VL (visceral leishmaniasis) most-affected populations. Credit: @ Don Paul-DNDi
The international research & development (R&D) consortium, AfriCoLeish, formed by six research organizations from East Africa and Europe, has launched a Phase III clinical study to address the extreme difficulty in treating visceral leishmaniasis (VL) in patients who also are HIV-positive.
The study will assess the efficacy and the safety of two treatments: a combination treatment of AmBisome® and miltefosine, and AmBisome® alone. This is the first randomized clinical trial in Africa to confirm the World Health Organization's recommendation for HIV-VL treatment. Two sites, Gondar and Abdurafi, in northwest Ethiopia, one of the highest burden areas in the world, have begun recruiting patients.
HIV-AIDS and VL, fatal without treatment, both affect the immune system of the patients. When the two diseases occur together, treatment of both diseases becomes more challenging. The risk of death from VL is nine times higher in patients who are co-infected with HIV. VL also accelerates the progression of HIV. Relapses of VL in patients co-infected with HIV are also very common, affecting half of treated patients within a year of initial treatment, and overall VL cure rates are significantly lower.
An emerging global problem, VL-HIV cases are reported in 35 countries worldwide, spanning Southern Europe, East Africa, the Indian subcontinent, and Latin America. One of the hardest hit areas in Africa is northwest Ethiopia, where anywhere from 20% to 40% of patients with VL were found to be also infected with HIV.
'Treating patients that suffer both HIV and visceral leishmaniasis is a real battle for clinicians. Research strongly suggests that we need to strike the right balance between stronger treatments and safer treatments', said Koert Ritmeijer, PhD, Health Advisor, Médecins Sans Frontières.
'Combining better initial cure treatments and preventing relapses will be a major step forward for our patients, who today fear that the diseases, which can be managed when they occur separately, often become a death sentence when they occur together. We have to do all we can to provide the most affected, a highly vulnerable migrant population, with an appropriate response', said Dr Ermias Diro, Principal Site Investigator, University of Gondar.
The Phase III clinical study conducted in Gondar and Abdurafi, Ethiopia, will assess the efficacy and safety of the combination of AmBisome® (30mg/kg total dose) and miltefosine (50mg or 100mg/day depending on the patient's weight), and AmBisome® alone (40mg/kg total dose). Total treatment duration is 28 days, if the tests show that the patient is parasite-negative (at day 29). Thereafter, the patient will start a secondary prophylaxis treatment aimed at preventing VL relapses and a one-year follow-up phase. A total of 132 patients will be recruited for the trial.
'Visceral leishmaniasis, notably in East Africa, is among the most neglected of all neglected tropical diseases. The work of this consortium to address the additional challenge of co-infection with HIV in Ethiopia is vital as it has begun to tackle one of the most challenging endemic areas and aspects of this fatal disease', said Dr Jorge Alvar, Head of Leishmanisis Programme, DNDi.
This clinical trial is conducted by DNDi (sponsor) in collaboration with MSF-Holland (co-sponsor); the University of Gondar (UoG), Ethiopia; the Institute of Tropical Medicine (ITM) Antwerp, Belgium; the London School of Hygiene & Tropical Medicine, UK; and is funded by the European Union (EU FP7); the Swiss Agency for Development and Cooperation (SDC), Switzerland; Médecins Sans Frontières; the Federal Ministry of Education and Research (BMBF) through KfW, Germany; the Medicor Foundation, Liechtenstein; and other private donors.
About the study:
- Overall objective: identify a safe and effective treatment for VL in HIV co-infected patients
- Study design: a randomized, parallel arm, open-label clinical study
- Treatment dosing schedule:
- AmBisome® monotherapy: 40mg/kg total dose: intravenous infusion 5mg/kg on day 1-5, 10, 17, 24
- AmBisome®: 30mg/kg total dose: intravenous infusion 5mg/kg on day 1, 3, 5, 7, 9, 11 and miltefosine: every day for 28 days, with 50mg/day (for patient weighting ≤ 25 kg) and 100 mg/day (for patient weighting > 25 kg)
- Study duration:
- Treatment duration is 28 days or 56 days in case of extended treatment (if tissue aspirate is parasite positive at day 29 and the patient is well). When the patients are tested parasite negative, they are eligible for a secondary prophylaxis – pentamidine 4mg/kg intramuscular injection or intravenous infusion once a month – and enter a one-year follow-up phase. At any stage as of day 29, if the patients are unwell, they receive a rescue therapy.
- AmBisome® supply for the study is donated by Gilead. Pentamidine supply for the study is donated by Sanofi.
About visceral leishmaniasis (VL or kala-azar)
Visceral leishmaniasis is a neglected tropical disease caused by Leishmania donovani or L. infantum. There are an estimated 300,000 new cases of VL per annum. Fatal if left untreated, the disease approximately kills 40,000 people every year. 90% of cases occur in Sudan, Ethiopia, Kenya, Bangladesh, India, Nepal, and Brazil. VL is characterized by prolonged fever and splenomegaly. The current VL therapies administered include pentavalent antimonials [sodium stibogluconate (SSG) and meglumine antimoniate], AmBisome®, miltefosine, and paromomycin (PM).
About AfriCoLeish: http://www.africoleish.org
A research and development (R&D) international consortium, AfriCoLeish is supported by the European Union Seventh Framework Programme (EU FP7) through a grant of €3 million. The project aims to test new treatments for visceral leishmaniasis (VL or kala-azar) in East Africa and co-infection of the disease with HIV in Ethiopia. AfriCoLeish brings together six organizations from Europe and East Africa with vast experience in R&D and treatment of HIV and kala-azar, namely:
- Drugs for Neglected Diseases initiative (DNDi): http://www.dndi.org
- Institute of Tropical Medicine (ITM) in Antwerp: http://www.itg.be
- London School of Hygiene & Tropical Medicine: http://www.lshtm.ac.uk
- Médecins Sans Frontières (MSF, The Netherlands): http://www.msf.org
- Institute of Endemic Diseases, University of Khartoum (IEND), Sudan: http://www.iend.edu.sd
- University of Gondar (UoG), Ethiopia: http://www.uog.edu.et
Press contact:
Renee Olende, Regional Communications Manager, DNDi Africa
Mobile: +254 705 639 909 / E-mail: rolende@dndi.org
Media Contact
All latest news from the category: Life Sciences and Chemistry
Articles and reports from the Life Sciences and chemistry area deal with applied and basic research into modern biology, chemistry and human medicine.
Valuable information can be found on a range of life sciences fields including bacteriology, biochemistry, bionics, bioinformatics, biophysics, biotechnology, genetics, geobotany, human biology, marine biology, microbiology, molecular biology, cellular biology, zoology, bioinorganic chemistry, microchemistry and environmental chemistry.
Newest articles

Why getting in touch with our ‘gerbil brain’ could help machines listen better
Macquarie University researchers have debunked a 75-year-old theory about how humans determine where sounds are coming from, and it could unlock the secret to creating a next generation of more…
Attosecond core-level spectroscopy reveals real-time molecular dynamics
Chemical reactions are complex mechanisms. Many different dynamical processes are involved, affecting both the electrons and the nucleus of the present atoms. Very often the strongly coupled electron and nuclear…
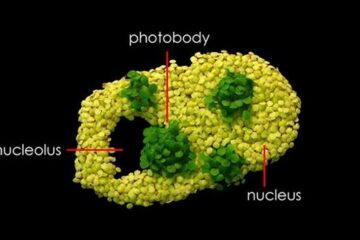
Free-forming organelles help plants adapt to climate change
Scientists uncover how plants “see” shades of light, temperature. Plants’ ability to sense light and temperature, and their ability to adapt to climate change, hinges on free-forming structures in their…