Study identifies how signals trigger cancer cells to spread
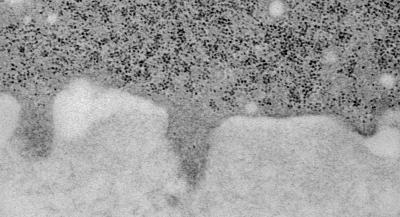
This electron micrograph shows a cancer cell (upper darker area) that has formed three invadopodia that are penetrating the adjacent extracellular matrix (lower lighter area). Credit: Jose Javier Bravo-Cordero/Albert Einstein College of Medicine
To migrate from a primary tumor, a cancer cell must first break through surrounding connective tissue known as the extracellular matrix (ECM). The cancer cell does so by forming short-lived invadopodia––foot-like protrusions these cells use to invade.
Invadopodia release enzymes that degrade the ECM, while other protrusions pull the cancer cell along, much like a locomotive pulls a train. The invading cancer cell relies on the cycle of invadopodium formation/disappearance to successfully travel from the tumor and enter nearby blood vessels to be carried to distant parts of the body.
“We've known for some time that invadopodia are driven by protein filaments called actin,” said study leader Louis Hodgson, Ph.D., assistant professor of anatomy and structural biology at Einstein. “But exactly what was regulating the actin in invadopodia was not clear.”
Previous studies had suggested that a protein called Rac1 played a role in cancer-cell invasion. When Rac1 levels are elevated, cancer cells display more invasive characteristics. But this suspected Rac1 activity in invadopodia had never been directly observed, only indirectly inferred.
To surmount this hurdle, Dr. Hodgson and his colleagues in the Gruss Lipper Biophotonics Center at Einstein devised a new fluorescent protein biosensor that, combined with live-cell imaging, revealed exactly when and where Rac1 is activated inside cancer cells.
Using this biosensor in highly invasive breast cancer cells taken from rodents and humans, the Einstein team discovered that when an individual invadopodium forms and is actively degrading the ECM, its Rac1 levels are low; on the other hand, elevated Rac1 levels coincide with the invadopodium's disappearance. “So high levels of Rac1 induce the disappearance of ECM-degrading invadopodia, while low levels allow them to stay—which is the complete opposite of what Rac1 was thought to be doing in invadopodia,” said Dr. Hodgson.
To confirm this observation, the researchers used siRNAs (molecules that silence gene expression) to turn off the RAC1 gene, which synthesizes Rac1 protein. When the gene was silenced, ECM degradation increased. Conversely, when Rac1 activity was enhanced—using light to activate a form of the Rac1 protein—the invadopodia disappeared.
In subsequent experiments, the Einstein team deciphered other parts of the Rac1 signaling cascade during invasion and showed that this signaling mechanism is regulated differently in normal breast epithelial cells.
“Rac1 levels in invadopodia of invasive tumor cells appear to surge and ebb at precisely timed intervals in order to maximize the cells' invasive capabilities,” said Dr. Hodgson.
Most of the 580,000 U.S. cancer deaths each year are caused by complications from the spread of cancer to distant tissues and organs, rather than from the primary tumor itself. So throwing a monkey wrench into the inner workings of invasive tumor cells—perhaps with a drug that prevents them from locally activating or inhibiting Rac1—could be extremely useful.
“Rac1 inhibitors have been developed,” Dr. Hodgson said, “but it wouldn't be safe to use them indiscriminately. Rac1 is an important molecule in healthy cells, including immune cells. So we'd need to find a way to shut off this signaling pathway specifically in cancer cells.”
The paper is titled “A Trio-Rac1-PAK1 signaling axis driving invadopodia disassembly.” The other contributors are: John Condeelis, Ph.D., Jose Javier Bravo-Cordero, Ph.D., and Ph.D. students Yasmin Moshfegh and Veronika Miskolci, all at Einstein.
The study was supported by grants from the National Institutes of Health (GM093121, T32GM007491, and CA150344).
The authors report no conflicts of interest.
About Albert Einstein College of Medicine of Yeshiva University
Albert Einstein College of Medicine of Yeshiva University is one of the nation's premier centers for research, medical education and clinical investigation. During the 2013-2014 academic year, Einstein is home to 734 M.D. students, 236 Ph.D. students, 106 students in the combined M.D./Ph.D. program, and 353 postdoctoral research fellows. The College of Medicine has more than 2,000 full-time faculty members located on the main campus and at its clinical affiliates. In 2013, Einstein received more than $155 million in awards from the NIH. This includes the funding of major research centers at Einstein in diabetes, cancer, liver disease, and AIDS. Other areas where the College of Medicine is concentrating its efforts include developmental brain research, neuroscience, cardiac disease, and initiatives to reduce and eliminate ethnic and racial health disparities. Its partnership with Montefiore Medical Center, the University Hospital and academic medical center for Einstein, advances clinical and translational research to accelerate the pace at which new discoveries become the treatments and therapies that benefit patients. Through its extensive affiliation network involving Montefiore, Jacobi Medical Center -Einstein's founding hospital, and five other hospital systems in the Bronx, Manhattan, Long Island and Brooklyn, Einstein runs one of the largest residency and fellowship training programs in the medical and dental professions in the United States. For more information, please visit www.einstein.yu.edu, read our blog, follow us on Twitter, like us on Facebook, and view us on YouTube.
Media Contact
More Information:
http://www.einstein.yu.edu/All latest news from the category: Life Sciences and Chemistry
Articles and reports from the Life Sciences and chemistry area deal with applied and basic research into modern biology, chemistry and human medicine.
Valuable information can be found on a range of life sciences fields including bacteriology, biochemistry, bionics, bioinformatics, biophysics, biotechnology, genetics, geobotany, human biology, marine biology, microbiology, molecular biology, cellular biology, zoology, bioinorganic chemistry, microchemistry and environmental chemistry.
Newest articles

Microscopic basis of a new form of quantum magnetism
Not all magnets are the same. When we think of magnetism, we often think of magnets that stick to a refrigerator’s door. For these types of magnets, the electronic interactions…

An epigenome editing toolkit to dissect the mechanisms of gene regulation
A study from the Hackett group at EMBL Rome led to the development of a powerful epigenetic editing technology, which unlocks the ability to precisely program chromatin modifications. Understanding how…

NASA selects UF mission to better track the Earth’s water and ice
NASA has selected a team of University of Florida aerospace engineers to pursue a groundbreaking $12 million mission aimed at improving the way we track changes in Earth’s structures, such…





















