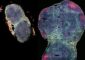
Scripps Research Institute Team Points to Brain’s ‘Dark Side’ as Key to Cocaine Addiction

In experiments with rats, the TSRI researchers found signs that cocaine-induced changes in this brain system contribute to anxiety-like behavior and other unpleasant symptoms of drug withdrawal—symptoms that typically drive an addict to keep using. When the researchers blocked specific brain receptors called kappa opioid receptors in this key anxiety-mediating brain region, the rats’ signs of addiction abated.
“These receptors appear to be a good target for therapy,” said Marisa Roberto, associate professor in TSRI’s addiction research group, the Committee on the Neurobiology of Addictive Disorders. Roberto was the principal investigator for the study, which appears in the journal Biological Psychiatry.
Carrot or Stick?
In addition to its clinical implications, the finding represents an alternative to the pleasure-seeking, “positive” motivational circuitry that is traditionally emphasized in addiction.
While changes in these pleasure-seeking brain networks may dominate the early period of drug use, scientists have been finding evidence of changes in the “negative” motivational circuitry as well—changes that move a person to take a drug not for its euphoric effects but for its (temporary) alleviation of the anxiety-ridden dysphoria of drug withdrawal. George F. Koob, chair of TSRI’s Committee on the Neurobiology of Addictive Disorders, has argued that these “dark side” brain changes mark the transition to a more persistent drug dependency.
In a series of recent studies, TSRI researchers including Roberto and Koob have highlighted the role of one of these dark-side actors: the receptor for the stress hormone CRF. Found abundantly in the central amygdala, CRF receptors become persistently overactive there as drug use increases, and that overactivity helps account for the negative symptoms of drug withdrawal.
The central amygdala also contains a high concentration of a class of neurotransmitters called dynorphins, which bind to kappa opioid receptors. Much like the CRF system, the dynorphin/kappa opioid system mediates negative, dysphoric feelings—and there have been hints from previous studies that CRF doesn’t work alone in producing such feelings during addiction.
“Our hypothesis was that the dynorphin/kappa opioid receptor system in the central amygdala also becomes overactive with excessive cocaine use,” said Marsida Kallupi, first author of the paper, who was a postdoctoral research associate in Roberto’s laboratory at the time of the study.
Such overactivity would be expected to arise as the brain struggles to maintain “reward homeostasis”—a middle-of-the-road balance between pleasure and displeasure—despite frequent drug-induced swerves toward euphoria. “Dynorphin possibly acts to balance the euphoric effects produced by other opioid systems during recreational drug use,” said Scott Edwards, who is a research associate in the Koob laboratory and a co-author of the study.
Reducing Signs of Addiction
When the TSRI researchers gave rats extended access to cocaine, the rats escalated their daily intake as many human users would. Sensitive electrophysiological measurements revealed signs of a persistent functional overactivity of the GABAergic system in the rats’ central amygdalae—which corresponds to an anxiety-like state in the animals. Probing with compounds that activate or block kappa opioid receptors, the scientists found signs that these receptors, like CRF receptors, do indeed help drive the central amygdala into overactivity during excessive cocaine use.
When the researchers blocked the kappa opioid receptors, central amygdala overactivity was greatly reduced. The same kappa opioid receptor-blocking treatment (antagonist) also reduced two standard signs of addiction in cocaine-using rats—the escalating hyperactive behavior each time the drug is taken and the anxiety-like behavior during withdrawal.
These results give Roberto and her colleagues hope that a similar treatment might help human cocaine addicts feel less compelled to keep using. Kappa opioid receptor blockers are already being developed for the treatment of depression and anxiety.
Blocking negative-motivational factors such as the kappa opioid and CRF systems also has the potential advantage that it spares the positive motivational pathways—the targets of older addiction therapies such as naltrexone. “We need to keep our positive motivational pathways intact so that they can signal the many normal rewarding events in our lives,” said Roberto. By contrast, she suspects, our negative motivational pathways involving CRF and kappa opioid receptors become abnormally active only in disease states such as addiction, and thus may be blocked more safely.
Other contributors to the study, “Kappa Opioid Receptor-Mediated Dysregulation of GABAergic Transmission in the Central Amygdala in Cocaine Addiction,” were Sunmee Wee of the Department of Molecular Therapeutics at TSRI’s Florida campus and Tim W. Whitfield Jr., Christopher S. Oleata, George Luu and Brooke E. Schmeichel of the Committee on the Neurobiology of Addictive Disorders at TSRI’s California campus.
The study was funded in part by grants from the National Institute on Alcohol Abuse and Alcoholism (AA020839, AA016895), the National Institute on Drug Abuse (DA025785, DA033726, DA004398) and The Pearson Center for Alcoholism and Addiction Research at TSRI.
About The Scripps Research Institute
The Scripps Research Institute (TSRI) is one of the world's largest independent, not-for-profit organizations focusing on research in the biomedical sciences. TSRI is internationally recognized for its contributions to science and health, including its role in laying the foundation for new treatments for cancer, rheumatoid arthritis, hemophilia, and other diseases. An institution that evolved from the Scripps Metabolic Clinic founded by philanthropist Ellen Browning Scripps in 1924, the institute now employs about 3,000 people on its campuses in La Jolla, CA, and Jupiter, FL, where its renowned scientists—including three Nobel laureates—work toward their next discoveries. The institute's graduate program, which awards PhD degrees in biology and chemistry, ranks among the top ten of its kind in the nation. For more information, see www.scripps.edu.
For information:
Office of Communications
Tel: 858-784-2666
Fax: 858-784-8136
press@scripps.edu
Media Contact
More Information:
http://www.scripps.eduAll latest news from the category: Life Sciences and Chemistry
Articles and reports from the Life Sciences and chemistry area deal with applied and basic research into modern biology, chemistry and human medicine.
Valuable information can be found on a range of life sciences fields including bacteriology, biochemistry, bionics, bioinformatics, biophysics, biotechnology, genetics, geobotany, human biology, marine biology, microbiology, molecular biology, cellular biology, zoology, bioinorganic chemistry, microchemistry and environmental chemistry.
Newest articles
Red light therapy for repairing spinal cord injury passes milestone
Patients with spinal cord injury (SCI) could benefit from a future treatment to repair nerve connections using red and near-infrared light. The method, invented by scientists at the University of…
Insect research is revolutionized by technology
New technologies can revolutionise insect research and environmental monitoring. By using DNA, images, sounds and flight patterns analysed by AI, it’s possible to gain new insights into the world of…

X-ray satellite XMM-newton sees ‘space clover’ in a new light
Astronomers have discovered enormous circular radio features of unknown origin around some galaxies. Now, new observations of one dubbed the Cloverleaf suggest it was created by clashing groups of galaxies….