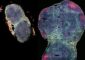
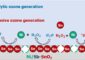
Discovery of New Family of Pseudo-Metallic Chemicals Changes

The periodic table of elements, all 111 of them, just got a little competition. A new discovery by a University of Missouri-Columbia research team, published in Angewandte Chemie, the journal of the German Society of Chemists, allows scientists to manipulate a molecule discovered 50 years ago in such as way as to give the molecule metal-like properties, creating a new, “pseudo” element. The pseudo-metal properties can be adjusted for a wide range of uses and might change the way scientists think about attacking disease or even building electronics.
Five decades ago, Fred Hawthorne, professor of radiology and director of the International Institute for Nano and Molecular Medicine at MU, discovered an extremely stable molecule consisting of 12 boron atoms and 12 hydrogen atoms. Known as “boron cages,” these molecules were difficult to change or manipulate, and sat dormant in Hawthorne's laboratory for many years.
Recently, Hawthorne's scientific team found a way to modify these cages, resulting in a large, new family of nano-sized compounds. In their study, which was published this month, Hawthorne, and Mark Lee, assistant professor at the institute and first author of the study, found that attaching different compounds to the cages gave them the properties of many different metals.
“Since the range of properties for these pseudo-metals is quite large, they might be referred to as 'psuedo-elements belonging to a completely new pseudo-periodic table,'” Lee said.
Potential applications of this discovery are abundant, especially in medicine.
“All living organisms are essentially a grand concert of chemical reactions involving the transfer of electrons between molecules and metals,” Lee said. “The electron transfer properties of this new family of molecules span the entire range of those found within living systems. Because of this, these pseudo-metals may be tuned for use as specific probes in living systems to detect or treat disease at the earliest state.”
In addition, because the compounds possess such a wide range of flexibility, they might have ramifications for nanotechnology and various kinds of electronics.
“This single discovery could open entirely new fields of study because of the controlled variability of the compounds,” Lee said. “We have the ability to change the properties of these pseudo-metals, which gives us the opportunity to tailor them to our needs, whether that is biomedical, chemical or electronic applications, some of which may utilize nanoscience.”
Media Contact
More Information:
http://www.missouri.eduAll latest news from the category: Life Sciences and Chemistry
Articles and reports from the Life Sciences and chemistry area deal with applied and basic research into modern biology, chemistry and human medicine.
Valuable information can be found on a range of life sciences fields including bacteriology, biochemistry, bionics, bioinformatics, biophysics, biotechnology, genetics, geobotany, human biology, marine biology, microbiology, molecular biology, cellular biology, zoology, bioinorganic chemistry, microchemistry and environmental chemistry.
Newest articles
Red light therapy for repairing spinal cord injury passes milestone
Patients with spinal cord injury (SCI) could benefit from a future treatment to repair nerve connections using red and near-infrared light. The method, invented by scientists at the University of…
Insect research is revolutionized by technology
New technologies can revolutionise insect research and environmental monitoring. By using DNA, images, sounds and flight patterns analysed by AI, it’s possible to gain new insights into the world of…

X-ray satellite XMM-newton sees ‘space clover’ in a new light
Astronomers have discovered enormous circular radio features of unknown origin around some galaxies. Now, new observations of one dubbed the Cloverleaf suggest it was created by clashing groups of galaxies….